当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Kinetic and DRIFTS studies of IrRu/Al2O3 catalysts for lean NOx reduction by CO at low temperature
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2020-11-10 , DOI: 10.1039/d0cy01835j Ji Hwan Song 1, 2, 3, 4, 5 , Dong Chan Park 1, 2, 3, 4, 5 , Young-Woo You 5, 6, 7, 8, 9 , Young Jin Kim 5, 6, 7, 8, 9 , Soo Min Kim 5, 6, 7, 8, 9 , Iljeong Heo 5, 6, 7, 8, 9 , Do Heui Kim 1, 2, 3, 4, 5
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2020-11-10 , DOI: 10.1039/d0cy01835j Ji Hwan Song 1, 2, 3, 4, 5 , Dong Chan Park 1, 2, 3, 4, 5 , Young-Woo You 5, 6, 7, 8, 9 , Young Jin Kim 5, 6, 7, 8, 9 , Soo Min Kim 5, 6, 7, 8, 9 , Iljeong Heo 5, 6, 7, 8, 9 , Do Heui Kim 1, 2, 3, 4, 5
Affiliation
|
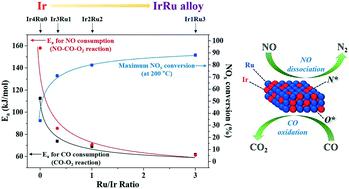
This study employs a series of bimetallic IrRu/Al2O3 catalysts with differing Ir:Ru compositions for lean NOx reduction by CO (CO-SCR). Catalytic activity assessments reveal that bimetallic IrRu/Al2O3 catalysts exhibit drastically enhanced de-NOx activities in the low-temperature region, compared with monometallic Ir/Al2O3 or Ru/Al2O3 catalysts. Furthermore, additional characterization and kinetic studies are used to elucidate the origin of the superior catalytic activity observed on bimetallic IrRu/Al2O3 catalysts. DRIFTS analyses show different adsorption affinities of the catalysts towards NO and CO, with CO for Ir and NO for Ru, respectively. The possibility of NCO intermediate formation is ruled out, which implies that the reduction of NOx proceeds through the dissociation of NO and the subsequent recombination of surface N species. Kinetic studies show that a monometallic Ir/Al2O3 catalyst favorably adsorbs CO, which acts as an inhibitor for facile NO adsorption and dissociation. However, bimetallic IrRu/Al2O3 catalysts exhibit higher affinity towards the adsorption of NO which, in terms of catalytic activities, would prove to be beneficial. Calculation of the activation energies for NO consumption and CO oxidation suggests that the rate determining step shifts from the dissociation of NO on a monometallic Ir/Al2O3 catalyst, to the removal of surface O with CO on bimetallic IrRu/Al2O3 catalysts. It is anticipated that the facile uptake of surface O through a CO oxidation reaction prevents the catalyst surface from being predominantly covered by CO or O species which could act as a poison, and leads to enhanced adsorption/dissociation of NO, resulting in a higher catalytic activity on the bimetallic IrRu/Al2O3 catalysts.
中文翻译:

IrRu / Al2O3催化剂在低温下通过CO还原贫NOx的动力学和DRIFTS研究
这项研究使用了一系列具有不同Ir:Ru组成的双金属IrRu / Al 2 O 3催化剂,用于通过CO(CO-SCR)还原稀薄的NO x。催化活性评估表明,与单金属Ir / Al 2 O 3或Ru / Al 2 O 3催化剂相比,双金属IrRu / Al 2 O 3催化剂在低温区域表现出显着增强的de-NO x活性。此外,还进行了其他表征和动力学研究,以阐明在双金属IrRu / Al 2 O 3上观察到的优异催化活性的起源。催化剂。DRIFTS分析显示催化剂对NO和CO的吸附亲和力不同,CO分别对Ir和NO对Ru的吸附。的NCO中间形成的可能性被排除了,这意味着NO的还原X通过NO的解离和面N物种的后续重组进行。动力学研究表明,单金属Ir / Al 2 O 3催化剂可很好地吸附CO,CO可作为便捷的NO吸附和离解的抑制剂。然而,双金属IrRu / Al 2 O 3催化剂对NO的吸附表现出更高的亲和力,就催化活性而言,这将被证明是有益的。NO消耗和CO氧化的活化能的计算表明,速率确定步骤从单金属Ir / Al 2 O 3催化剂上的NO分解,转变为双金属IrRu / Al 2 O 3上的CO去除表面O。催化剂。可以预料的是,通过CO氧化反应容易吸收表面O,可防止催化剂表面主要被可能起毒作用的CO或O所覆盖,并导致NO的吸附/离解增加,从而提高催化活性。活性对IrRu / Al 2 O双金属的影响3种催化剂。
更新日期:2020-11-21
中文翻译:

IrRu / Al2O3催化剂在低温下通过CO还原贫NOx的动力学和DRIFTS研究
这项研究使用了一系列具有不同Ir:Ru组成的双金属IrRu / Al 2 O 3催化剂,用于通过CO(CO-SCR)还原稀薄的NO x。催化活性评估表明,与单金属Ir / Al 2 O 3或Ru / Al 2 O 3催化剂相比,双金属IrRu / Al 2 O 3催化剂在低温区域表现出显着增强的de-NO x活性。此外,还进行了其他表征和动力学研究,以阐明在双金属IrRu / Al 2 O 3上观察到的优异催化活性的起源。催化剂。DRIFTS分析显示催化剂对NO和CO的吸附亲和力不同,CO分别对Ir和NO对Ru的吸附。的NCO中间形成的可能性被排除了,这意味着NO的还原X通过NO的解离和面N物种的后续重组进行。动力学研究表明,单金属Ir / Al 2 O 3催化剂可很好地吸附CO,CO可作为便捷的NO吸附和离解的抑制剂。然而,双金属IrRu / Al 2 O 3催化剂对NO的吸附表现出更高的亲和力,就催化活性而言,这将被证明是有益的。NO消耗和CO氧化的活化能的计算表明,速率确定步骤从单金属Ir / Al 2 O 3催化剂上的NO分解,转变为双金属IrRu / Al 2 O 3上的CO去除表面O。催化剂。可以预料的是,通过CO氧化反应容易吸收表面O,可防止催化剂表面主要被可能起毒作用的CO或O所覆盖,并导致NO的吸附/离解增加,从而提高催化活性。活性对IrRu / Al 2 O双金属的影响3种催化剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号