当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Entropy driven preference for alkene adsorption at the pore mouth as the origin of pore-mouth catalysis for alkane hydroisomerization in 1D zeolites
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2020-11-03 , DOI: 10.1039/d0cy01485k Aleksandr A. Shubin 1, 2, 2, 3, 4 , Igor L. Zilberberg 1, 2, 2, 3, 4
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2020-11-03 , DOI: 10.1039/d0cy01485k Aleksandr A. Shubin 1, 2, 2, 3, 4 , Igor L. Zilberberg 1, 2, 2, 3, 4
Affiliation
|
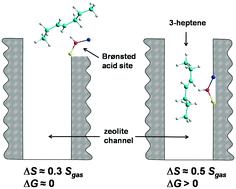
The hydroisomerization of n-paraffins to mono-methyl branched isomers on bifunctional metal acid-zeolite catalysts has been commonly considered in terms of the heuristic pore-mouth catalysis model developed to explain the highly selective formation of the monobranched isomer with the methyl group at the C2 position. This work presents a theoretical support of the pore-mouth model on the basis of semi-quantitative estimates of the entropy change upon adsorption at the opening of the zeolite channel and inside the pore, and the DFT calculated enthalpy for 3-heptene adsorption on the ZSM-23 zeolite. A key prediction is the entropy-driven preference for alkene (assumed to be readily produced by metal particle on the zeolite surface) to adsorb and isomerize only at the mouth of the zeolite pore being trapped by the Brønsted acid site via the alkene double bond located near the end of the molecule. This effect explains the origin of the pore-mouth catalysis and positional selectivity of the skeletal isomerization.
中文翻译:

熵驱动的孔口烯烃吸附的偏好是一维沸石中烷烃加氢异构化的孔口催化的起源
n的加氢异构化在开发用于解释在C2位置带有甲基的单支链异构体的高度选择性形成的启发式孔口催化模型方面,通常考虑将双官能金属酸-沸石催化剂上的链烷烃转化为单甲基支链异构体。这项工作基于对沸石通道开口处和孔内吸附时熵变化的半定量估计,以及DFT计算的3-庚烯吸附焓,为孔口模型提供了理论支持。 ZSM-23沸石。一个关键的预测是熵驱动的偏爱烯烃(假定是由沸石表面的金属颗粒容易产生的)仅在被布朗斯台德酸位点俘获的沸石孔口处吸附和异构化。位于分子末端附近的烯烃双键。该效应解释了孔口催化的起源和骨架异构化的位置选择性。
更新日期:2020-11-21
中文翻译:

熵驱动的孔口烯烃吸附的偏好是一维沸石中烷烃加氢异构化的孔口催化的起源
n的加氢异构化在开发用于解释在C2位置带有甲基的单支链异构体的高度选择性形成的启发式孔口催化模型方面,通常考虑将双官能金属酸-沸石催化剂上的链烷烃转化为单甲基支链异构体。这项工作基于对沸石通道开口处和孔内吸附时熵变化的半定量估计,以及DFT计算的3-庚烯吸附焓,为孔口模型提供了理论支持。 ZSM-23沸石。一个关键的预测是熵驱动的偏爱烯烃(假定是由沸石表面的金属颗粒容易产生的)仅在被布朗斯台德酸位点俘获的沸石孔口处吸附和异构化。位于分子末端附近的烯烃双键。该效应解释了孔口催化的起源和骨架异构化的位置选择性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号