当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Confinement-Enhanced Luminescence in Protein–Gold Nanoclusters
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2020-11-20 , DOI: 10.1021/acs.jpclett.0c03054 Paul Roberts , Jeneh Karima Perry 1 , Raj K. Gupta 2 , Shashi P. Karna 1 , Joelle Frechette
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2020-11-20 , DOI: 10.1021/acs.jpclett.0c03054 Paul Roberts , Jeneh Karima Perry 1 , Raj K. Gupta 2 , Shashi P. Karna 1 , Joelle Frechette
Affiliation
|
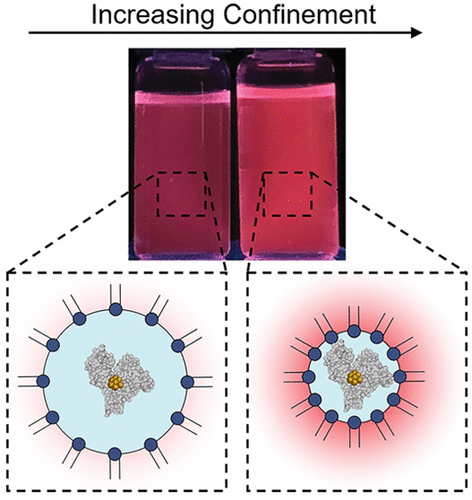
Confinement has profound effects on protein functions. Nanoscale probes for confinement or excluded volume interactions could help us understand how these interactions influence protein functions. This work reports on the increased luminescence of BSA–gold nanoclusters when confined. Confinement of the BSA–gold nanoclusters occurred within reverse micelles (RMs), where the size of the RMs determined the degree of confinement. The confinement-enhanced luminescence is reversible, i.e., the emission returns to its original value following cyclic changes in RM size. Circular dichroism measurements show an increase in alpha-helical character of the BSA-stabilized nanoclusters with confinement, which could provide a mechanism for the increase in luminescence. The alpha-helical character of the native proteins also increases with confinement, suggesting that the protein–nanocluster might sense confinement in an analogous fashion as the proteins. When the RMs approach the size of the protein, the intensity becomes independent of alpha-helical character, suggesting a different mechanism for the luminescence increase.
中文翻译:

蛋白质-金纳米簇的禁闭增强发光
封闭对蛋白质功能有深远的影响。用于限制或排除体积相互作用的纳米探针可以帮助我们了解这些相互作用如何影响蛋白质功能。这项工作报告了封闭时BSA-金纳米团簇的发光增加。BSA-金纳米团簇的封闭发生在反胶束(RM)中,RM的大小决定了封闭的程度。限制增强的发光是可逆的,即,随着RM大小的周期性变化,发射返回其原始值。圆形二向色性测量表明,BSA稳定的纳米团簇的α-螺旋特性随空间的增加而增加,这可以提供增加发光的机制。天然蛋白质的α-螺旋特性也随限制而增加,这表明蛋白质纳米簇可能以与蛋白质类似的方式感觉到限制。当RM接近蛋白质的大小时,强度变得与α螺旋特征无关,这表明了不同的发光增加机理。
更新日期:2020-12-03
中文翻译:

蛋白质-金纳米簇的禁闭增强发光
封闭对蛋白质功能有深远的影响。用于限制或排除体积相互作用的纳米探针可以帮助我们了解这些相互作用如何影响蛋白质功能。这项工作报告了封闭时BSA-金纳米团簇的发光增加。BSA-金纳米团簇的封闭发生在反胶束(RM)中,RM的大小决定了封闭的程度。限制增强的发光是可逆的,即,随着RM大小的周期性变化,发射返回其原始值。圆形二向色性测量表明,BSA稳定的纳米团簇的α-螺旋特性随空间的增加而增加,这可以提供增加发光的机制。天然蛋白质的α-螺旋特性也随限制而增加,这表明蛋白质纳米簇可能以与蛋白质类似的方式感觉到限制。当RM接近蛋白质的大小时,强度变得与α螺旋特征无关,这表明了不同的发光增加机理。











































 京公网安备 11010802027423号
京公网安备 11010802027423号