当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stereoselective Construction of the Methylcyclopentane Core of Peditithins B–H with Five Continuous Stereocenters
Organic Letters ( IF 4.9 ) Pub Date : 2020-11-20 , DOI: 10.1021/acs.orglett.0c03615 Fu-Qiang Ni 1 , Shu-Qi Wu 1 , Wei Li 1 , Qingjiang Li 1 , Sheng Yin 1
Organic Letters ( IF 4.9 ) Pub Date : 2020-11-20 , DOI: 10.1021/acs.orglett.0c03615 Fu-Qiang Ni 1 , Shu-Qi Wu 1 , Wei Li 1 , Qingjiang Li 1 , Sheng Yin 1
Affiliation
|
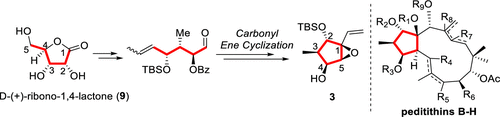
A stereoselective construction of the methylcyclopentane core (3) of jatrophane diterpenoids peditithins B–H was achieved in 14 steps from commercially available d-(+)-ribono-1,4-lactone (9). The linear 5-ene-heptanal derived from 9 was cyclized to the five-membered ring by an intramolecular carbonyl ene reaction, and five continuous stereocenters on 3 were stereoselectively introduced via a successive substrate-controlled manner, involving diastereoselective 1,4-addition, MoOPH-induced hydroxylation, and stereospecific epoxidation.
中文翻译:

具有五个连续立体中心的Peditithins B–H甲基环戊烷核的立体选择性构建
从市售的d -(+)-ribono-1,4-内酯(9)分离14步,即可实现麻疯树二萜类吡啶酮BH的甲基环戊烷核心(3)的立体选择性构建。通过分子内羰基烯反应将9衍生的线性5烯-庚醛环化成五元环,并通过连续的底物控制方式立体选择性地引入3上的5个连续立体中心,涉及非对映选择性的1,4-加成, MoOPH诱导的羟基化和立体定向环氧化。
更新日期:2020-12-04
中文翻译:

具有五个连续立体中心的Peditithins B–H甲基环戊烷核的立体选择性构建
从市售的d -(+)-ribono-1,4-内酯(9)分离14步,即可实现麻疯树二萜类吡啶酮BH的甲基环戊烷核心(3)的立体选择性构建。通过分子内羰基烯反应将9衍生的线性5烯-庚醛环化成五元环,并通过连续的底物控制方式立体选择性地引入3上的5个连续立体中心,涉及非对映选择性的1,4-加成, MoOPH诱导的羟基化和立体定向环氧化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号