当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selectively Oxidative C(sp2)–H/C(sp3)–H Cross-Coupling of Benzamides with Amides by Nickel Catalysis
Organic Letters ( IF 4.9 ) Pub Date : 2020-11-19 , DOI: 10.1021/acs.orglett.0c03535 Ningning Lv 1 , Shuling Yu 2 , Chao Hong 2 , De-Man Han 3 , Yuhong Zhang 2
Organic Letters ( IF 4.9 ) Pub Date : 2020-11-19 , DOI: 10.1021/acs.orglett.0c03535 Ningning Lv 1 , Shuling Yu 2 , Chao Hong 2 , De-Man Han 3 , Yuhong Zhang 2
Affiliation
|
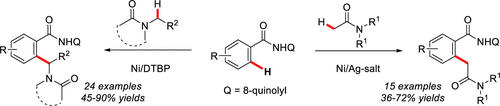
The oxidative cross-coupling between the α-C(sp3)–H bond of amide in DMAc and the inert ortho-C(sp2)–H bond of benzamides is achieved for the first time by nickel catalysis, with the assistance of 8-aminoquinolyl group in the presence of a silver oxidant. Notably, the selectivity of conversion can be perfectly controlled by modulating the oxidant additives, and the products from the coupling of the C(sp3)–H bond adjacent to nitrogen of amides with benzamides are approached through the use of peroxide.
中文翻译:

镍催化苯甲酰胺与酰胺的选择性氧化C(sp 2)–H / C(sp 3)–H交叉偶联
在DMAc的帮助下,镍催化首次实现了DMAc中酰胺的α-C(sp 3)-H键与苯甲酰胺的惰性邻-C(sp 2)-H键之间的氧化交叉偶联。在银氧化剂存在下的8-氨基喹啉基。值得注意的是,可以通过调节氧化剂的添加来完美地控制转化的选择性,并且通过使用过氧化物可以接近与酰胺氮相邻的C(sp 3)-H键与苯甲酰胺偶合的产物。
更新日期:2020-12-04
中文翻译:

镍催化苯甲酰胺与酰胺的选择性氧化C(sp 2)–H / C(sp 3)–H交叉偶联
在DMAc的帮助下,镍催化首次实现了DMAc中酰胺的α-C(sp 3)-H键与苯甲酰胺的惰性邻-C(sp 2)-H键之间的氧化交叉偶联。在银氧化剂存在下的8-氨基喹啉基。值得注意的是,可以通过调节氧化剂的添加来完美地控制转化的选择性,并且通过使用过氧化物可以接近与酰胺氮相邻的C(sp 3)-H键与苯甲酰胺偶合的产物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号