当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Experimental Study of Carbon Dioxide Solubility in Sodium Chloride and Calcium Chloride Brines at 333.15 and 453.15 K for Pressures up to 40 MPa
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-11-20 , DOI: 10.1021/acs.jced.0c00592 José Lara Cruz 1 , Esther Neyrolles 1 , François Contamine 1 , Pierre Cézac 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-11-20 , DOI: 10.1021/acs.jced.0c00592 José Lara Cruz 1 , Esther Neyrolles 1 , François Contamine 1 , Pierre Cézac 1
Affiliation
|
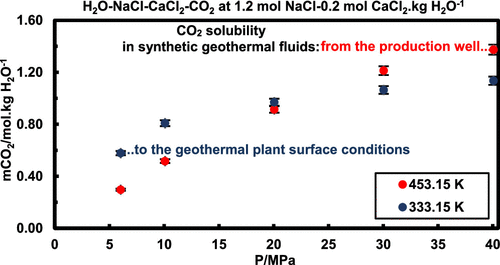
As part of the global efforts for renewable energy development, carbon dioxide solubility measurements were provided for the geothermal industry. Solubility is obtained at high pressures (6–40 MPa), in aqueous phases reproducing the exploited geothermal resources, through sodium chloride (NaCl) and calcium chloride (CaCl2) synthetic brines (1.2 mol NaCl·kg H2O–1 and 0.2 mol CaCl2·kg H2O–1), at 333.15 and 453.15 K. Both mixed-salt and single-salt brines were analyzed to produce original results. A new stirred reactor, with variable volume, was conceived to maintain gas–liquid equilibria, while solubility measurements were performed by titration, with a relative uncertainty of 0.028. The experimental methods were validated with solubility analyses in pure water, at 333.15 K, and in sodium chloride and calcium chloride single-salt brines (1 mol·kg H2O–1), at 323.15 K. Then, 20 original data were obtained and compared to PHREEQC (pitzer.dat) calculation results. The measurements highlight that carbon dioxide solubility decreases when the aqueous phase salinity increases. This salting-out effect increases to about 23%, when observed in the analyzed mixed-salt brines at 333.15 K, and it is mostly caused by sodium chloride in these fluids. However, one can notice that calcium chloride in these brines promotes part of the phenomenon too.
中文翻译:

压力高达40 MPa时333.15和453.15 K下二氧化碳在氯化钠和氯化钙中的溶解度的实验研究
作为全球可再生能源开发工作的一部分,为地热行业提供了二氧化碳溶解度测量。在高压(6– 40 MPa)的水相中,通过氯化钠(NaCl)和氯化钙(CaCl 2)合成盐水(1.2 mol NaCl·kg H 2 O –1和0.2 )获得溶解度,该水相可再生利用的地热资源。摩尔CaCl 2 ·kg H 2 O –1),分别在333.15和453.15 K处进行了分析。混合盐和单盐盐水均经过分析以产生原始结果。设计了一个新的具有可变体积的搅拌反应器,以保持气-液平衡,而溶解度测量是通过滴定进行的,相对不确定度为0.028。通过在333.15 K纯水以及氯化钠和氯化钙单盐盐水(1 mol·kg H 2 O –1),在323.15K。然后,获得20个原始数据,并将其与PHREEQC(pitzer.dat)计算结果进行比较。测量结果表明,当水相盐度增加时,二氧化碳溶解度会降低。在分析的333.15 K的混合盐盐水中观察到盐析效果增加到约23%,这主要是由这些流体中的氯化钠引起的。但是,人们可以注意到,这些盐水中的氯化钙也促进了部分现象。
更新日期:2021-01-14
中文翻译:

压力高达40 MPa时333.15和453.15 K下二氧化碳在氯化钠和氯化钙中的溶解度的实验研究
作为全球可再生能源开发工作的一部分,为地热行业提供了二氧化碳溶解度测量。在高压(6– 40 MPa)的水相中,通过氯化钠(NaCl)和氯化钙(CaCl 2)合成盐水(1.2 mol NaCl·kg H 2 O –1和0.2 )获得溶解度,该水相可再生利用的地热资源。摩尔CaCl 2 ·kg H 2 O –1),分别在333.15和453.15 K处进行了分析。混合盐和单盐盐水均经过分析以产生原始结果。设计了一个新的具有可变体积的搅拌反应器,以保持气-液平衡,而溶解度测量是通过滴定进行的,相对不确定度为0.028。通过在333.15 K纯水以及氯化钠和氯化钙单盐盐水(1 mol·kg H 2 O –1),在323.15K。然后,获得20个原始数据,并将其与PHREEQC(pitzer.dat)计算结果进行比较。测量结果表明,当水相盐度增加时,二氧化碳溶解度会降低。在分析的333.15 K的混合盐盐水中观察到盐析效果增加到约23%,这主要是由这些流体中的氯化钠引起的。但是,人们可以注意到,这些盐水中的氯化钙也促进了部分现象。











































 京公网安备 11010802027423号
京公网安备 11010802027423号