当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Measurement and Correlation of Solubility of Methylsalicylic Acid Isomers in Supercritical Carbon Dioxide
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-11-20 , DOI: 10.1021/acs.jced.0c00647 Shin-Wei Wang, Jhih-Zong Chen, Chieh-Ming Hsieh
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-11-20 , DOI: 10.1021/acs.jced.0c00647 Shin-Wei Wang, Jhih-Zong Chen, Chieh-Ming Hsieh
|
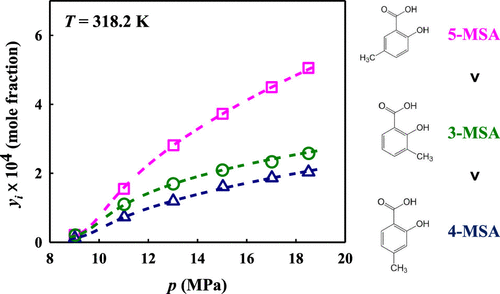
A semiflow type apparatus for solid solute solubility measurement was constructed to investigate the solubility of isomers of methylsalicylic acid (MSA) in supercritical carbon dioxide (ScCO2). The solubility of salicylic acid in ScCO2 was measured in seeking to validate the reliability of the apparatus and the experimental procedure. The solubility of three MSA isomers, 3-MSA, 4-MSA, and 5-MSA, were measured at 308.2, 318.2, and 328.2 K, respectively, over a pressure range of 9–18.5 MPa. The results showed that the solubility of MSA isomers was within the range of 8.3 × 10–6 and 5.9 × 10–4. These newly measured solubility data were correlated by four semiempirical models; satisfactory correlation results were obtained with the average absolute relative deviation in solubility within 8.17%. The consistency of the solubility data was confirmed by the good correlation results with the Chrastil and Méndez-Santiago and Teja models.
中文翻译:

甲基水杨酸异构体在超临界二氧化碳中的溶解度测定与相关
构造了用于固体溶质溶解度测量的半流式装置,以研究甲基水杨酸(MSA)异构体在超临界二氧化碳(ScCO 2)中的溶解度。测量水杨酸在ScCO 2中的溶解度,以试图验证设备和实验程序的可靠性。在9-18.5 MPa的压力范围内,分别测量了三种MSA异构体3-MSA,4-MSA和5-MSA的溶解度,分别为308.2 K,318.2 K和328.2K。结果表明,MSA异构体的溶解度在8.3×10 –6到5.9×10 –4的范围内。这些新测得的溶解度数据通过四个半经验模型进行了关联。获得了令人满意的相关结果,溶解度的平均绝对相对偏差在8.17%之内。与Chrastil和Méndez-Santiago和Teja模型的良好相关性结果证实了溶解度数据的一致性。
更新日期:2021-01-14
中文翻译:

甲基水杨酸异构体在超临界二氧化碳中的溶解度测定与相关
构造了用于固体溶质溶解度测量的半流式装置,以研究甲基水杨酸(MSA)异构体在超临界二氧化碳(ScCO 2)中的溶解度。测量水杨酸在ScCO 2中的溶解度,以试图验证设备和实验程序的可靠性。在9-18.5 MPa的压力范围内,分别测量了三种MSA异构体3-MSA,4-MSA和5-MSA的溶解度,分别为308.2 K,318.2 K和328.2K。结果表明,MSA异构体的溶解度在8.3×10 –6到5.9×10 –4的范围内。这些新测得的溶解度数据通过四个半经验模型进行了关联。获得了令人满意的相关结果,溶解度的平均绝对相对偏差在8.17%之内。与Chrastil和Méndez-Santiago和Teja模型的良好相关性结果证实了溶解度数据的一致性。









































 京公网安备 11010802027423号
京公网安备 11010802027423号