当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rational Ligand Design Enables pH Control over Aqueous Iron Magnetostructural Dynamics and Relaxometric Properties
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2020-11-20 , DOI: 10.1021/acs.inorgchem.0c02923 Huan Wang , Alison Wong , Luke C. Lewis 1 , Genevieve R. Nemeth , Veronica Clavijo Jordan , Jeffrey W. Bacon 2 , Peter Caravan , Hannah S. Shafaat 1 , Eric M. Gale
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2020-11-20 , DOI: 10.1021/acs.inorgchem.0c02923 Huan Wang , Alison Wong , Luke C. Lewis 1 , Genevieve R. Nemeth , Veronica Clavijo Jordan , Jeffrey W. Bacon 2 , Peter Caravan , Hannah S. Shafaat 1 , Eric M. Gale
Affiliation
|
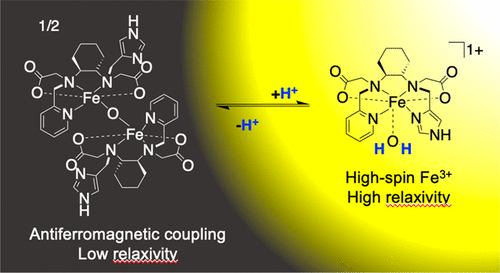
Complexes of Fe3+ engage in rich aqueous solution speciation chemistry in which discrete molecules can react with solvent water to form multinuclear μ-oxo and μ-hydroxide bridged species. Here we demonstrate how pH- and concentration-dependent equilibration between monomeric and μ-oxo-bridged dimeric Fe3+ complexes can be controlled through judicious ligand design. We purposed this chemistry to develop a first-in-class Fe3+-based MR imaging probe, Fe-PyCy2AI, that undergoes relaxivity change via pH-mediated control of monomer vs dimer speciation. The monomeric complex exists in a S = 5/2 configuration capable of inducing efficient T1-relaxation, whereas the antiferromagnetically coupled dimeric complex is a much weaker relaxation agent. The mechanisms underpinning the pH dependence on relaxivity were interrogated by using a combination of pH potentiometry, 1H and 17O relaxometry, electronic absorption spectroscopy, bulk magnetic susceptibility, electron paramagnetic resonance spectroscopy, and X-ray crystallography measurements. Taken together, the data demonstrate that PyCy2AI forms a ternary complex with high-spin Fe3+ and a rapidly exchanging water coligand, [Fe(PyCy2AI)(H2O)]+ (ML), which can deprotonate to form the high-spin complex [Fe(PyCy2AI)(OH)] (ML(OH)). Under titration conditions of 7 mM Fe complex, water coligand deprotonation occurs with an apparent pKa 6.46. Complex ML(OH) dimerizes to form the antiferromagnetically coupled dimeric complex [(Fe(PyCy2AI))2O] ((ML)2O) with an association constant (Ka) of 5.3 ± 2.2 mM–1. The relaxivity of the monomeric complexes are between 7- and 18-fold greater than the antiferromagnetically coupled dimer at applied field strengths ranging between 1.4 and 11.7 T. ML(OH) and (ML)2O interconvert rapidly within the pH 6.0–7.4 range that is relevant to human pathophysiology, resulting in substantial observed relaxivity change. Controlling Fe3+ μ-oxo bridging interactions through rational ligand design and in response to local chemical environment offers a robust mechanism for biochemically responsive MR signal modulation.
中文翻译:

合理的配体设计实现了对铁水结构动力学和弛豫特性的pH控制
Fe 3+的络合物参与了丰富的水溶液形态化学反应,其中离散的分子可以与溶剂水反应形成多核μ-氧代和μ-羟基桥接物种。在这里,我们证明了如何通过明智的配体设计来控制单体和μ-氧代桥联的二聚Fe 3+配合物之间的pH和浓度依赖性平衡。我们打算用这种化学方法开发出一流的基于Fe 3+的MR成像探针Fe-PyCy2AI,该探针通过pH介导的单体对二聚体形成的控制而经历弛豫度变化。单体络合物以S = 5/2构型存在,能够诱导有效的T 1松弛,而反铁磁偶联的二聚体复合物则弱得多。通过使用pH电位法,1 H和17 O弛豫法,电子吸收光谱法,体磁化率,电子顺磁共振光谱法和X射线晶体学测量法的组合,来质疑支撑pH依赖于弛豫性的机制。两者合计,数据表明PyCy2AI与高自旋Fe 3+和快速交换的水大肠菌群[Fe(PyCy2AI)(H 2 O)] +(ML)形成三元复合物,可以去质子化形成高-自旋复合物[Fe(PyCy2Al)(OH)](ML(OH))。在7 mM Fe络合物的滴定条件下,水大肠菌质的去质子化作用的表观p K a为6.46。配合物ML(OH)二聚形成反铁磁偶联的二聚体配合物[(Fe(PyCy2AI)2 O]((ML)2 O),缔合常数(K a)为5.3±2.2 mM –1。在1.4至11.7 T范围内施加的场强下,单体配合物的弛豫度比反铁磁耦合二聚体大7至18倍。ML(OH)和(ML)2 O在与人类病理生理相关的pH 6.0-7.4范围内,它们可以快速相互转化,从而导致观察到的弛豫度变化很大。通过合理的配体设计和对局部化学环境的反应来控制Fe 3+ μ-氧代桥联反应,为生化响应性MR信号调制提供了强大的机制。
更新日期:2020-12-07
中文翻译:

合理的配体设计实现了对铁水结构动力学和弛豫特性的pH控制
Fe 3+的络合物参与了丰富的水溶液形态化学反应,其中离散的分子可以与溶剂水反应形成多核μ-氧代和μ-羟基桥接物种。在这里,我们证明了如何通过明智的配体设计来控制单体和μ-氧代桥联的二聚Fe 3+配合物之间的pH和浓度依赖性平衡。我们打算用这种化学方法开发出一流的基于Fe 3+的MR成像探针Fe-PyCy2AI,该探针通过pH介导的单体对二聚体形成的控制而经历弛豫度变化。单体络合物以S = 5/2构型存在,能够诱导有效的T 1松弛,而反铁磁偶联的二聚体复合物则弱得多。通过使用pH电位法,1 H和17 O弛豫法,电子吸收光谱法,体磁化率,电子顺磁共振光谱法和X射线晶体学测量法的组合,来质疑支撑pH依赖于弛豫性的机制。两者合计,数据表明PyCy2AI与高自旋Fe 3+和快速交换的水大肠菌群[Fe(PyCy2AI)(H 2 O)] +(ML)形成三元复合物,可以去质子化形成高-自旋复合物[Fe(PyCy2Al)(OH)](ML(OH))。在7 mM Fe络合物的滴定条件下,水大肠菌质的去质子化作用的表观p K a为6.46。配合物ML(OH)二聚形成反铁磁偶联的二聚体配合物[(Fe(PyCy2AI)2 O]((ML)2 O),缔合常数(K a)为5.3±2.2 mM –1。在1.4至11.7 T范围内施加的场强下,单体配合物的弛豫度比反铁磁耦合二聚体大7至18倍。ML(OH)和(ML)2 O在与人类病理生理相关的pH 6.0-7.4范围内,它们可以快速相互转化,从而导致观察到的弛豫度变化很大。通过合理的配体设计和对局部化学环境的反应来控制Fe 3+ μ-氧代桥联反应,为生化响应性MR信号调制提供了强大的机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号