当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Inception of Co3O4 as Microstructural Support to Promote Alkaline Oxygen Evolution Reaction for Co0.85Se/Co9Se8 Network
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2020-11-19 , DOI: 10.1021/acs.inorgchem.0c02618 Sourav Ghosh 1 , Gouri Tudu 1 , Ayan Mondal 1 , Sagar Ganguli 1 , Harish Reddy Inta 1 , Venkataramanan Mahalingam 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2020-11-19 , DOI: 10.1021/acs.inorgchem.0c02618 Sourav Ghosh 1 , Gouri Tudu 1 , Ayan Mondal 1 , Sagar Ganguli 1 , Harish Reddy Inta 1 , Venkataramanan Mahalingam 1
Affiliation
|
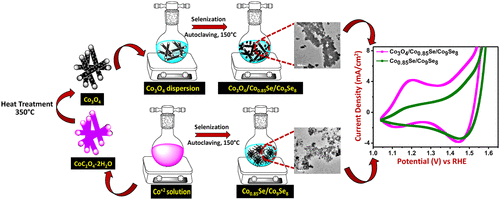
Developing electrocatalysts with abundant active sites is a substantial challenge to reduce the overpotential requirement for the alkaline oxygen evolution reaction (OER). In this work, we have aimed to improve the catalytic activity of cobalt selenides by growing them over the self-supported Co3O4 microrods. Initially, Co3O4 microrods were synthesized through annealing of an as-prepared cobalt oxalate precursor. The subsequent selenization of Co3O4 resulted in the formation of a grainy rodlike Co3O4/Co0.85Se/Co9Se8 network. The structural and morphological analysis reveals the presence of Co3O4 even after the selenization treatment where the cobalt selenide nanograins are randomly covered over the Co3O4 support. The resultant electrode shows superior electrocatalytic activity toward OER in alkaline medium by delivering a benchmark current density of 10 mA/cm2geo at an overpotential of 330 mV. As a comparison, we have developed Co0.85Se/Co9Se8 under similar conditions and evaluated its OER activity. This material consumes an overpotential of 360 mV to deliver the benchmark current density, which signifies the role of the Co3O4 support to improve the electrocatalytic activity of Co0.85Se/Co9Se8. Despite having a low TOF value for Co3O4/Co0.85Se/Co9Se8 (0.0076 s–1) compared to Co0.85Se/Co9Se8 (0.0102 s–1), the improved catalytic activity of Co3O4/Co0.85Se/Co9Se8 is attributed to the presence of a higher number of active sites rather than the improved per site activity. This is further supported from the Cdl (double layer capacitance) measurements where Co3O4/Co0.85Se/Co9Se8 and Co0.85Se/Co9Se8 tender Cdl values of about 8.19 and 1.08 mF/cm2, respectively, after electrochemical precondition. As-prepared Co3O4/Co0.85Se/Co9Se8 also manifests rapid kinetics (low Tafel slope ∼ 91 mV/dec), long-term stability, low charge-transfer resistance, and 82% Faradaic efficiency for alkaline electrocatalysis (pH = 14). Furthermore, the proton reaction order (ρRHE) is found to be 0.65, indicating a proton decoupled electron transfer (PDET) mechanism for alkaline OER. Thus, the Co3O4 support helps in the exposure of more catalytic sites of Co0.85Se/Co9Se8 to deliver the improved catalytic activities in alkaline medium.
中文翻译:

Co 3 O 4作为微结构载体的引入,以促进Co 0.85 Se / Co 9 Se 8网络的碱性氧释放反应
开发具有丰富活性位点的电催化剂是降低碱性氧放出反应(OER)的过电位要求的一项重大挑战。在这项工作中,我们旨在通过使硒化钴在自支撑的Co 3 O 4微棒上生长来提高其催化活性。最初,通过对所制备的草酸钴前体进行退火来合成Co 3 O 4微棒。随后的Co 3 O 4硒化导致形成粒状棒状Co 3 O 4 / Co 0.85 Se / Co 9 Se 8网络。即使在硒化处理之后,硒化钴纳米颗粒被随机覆盖在Co 3 O 4载体上,结构和形态分析也显示出Co 3 O 4的存在。通过在330 mV的超电势下提供10 mA / cm 2 geo的基准电流密度,所得电极在碱性介质中对OER具有优异的电催化活性。作为比较,我们在相似的条件下开发了Co 0.85 Se / Co 9 Se 8并评估了其OER活性。该材料消耗360 mV的过电势以提供基准电流密度,这表明Co的作用3 O 4载体可改善Co 0.85 Se / Co 9 Se 8的电催化活性。尽管具有低TOF值钴3 ö 4 /钴0.85硒/钴9硒8(0.0076小号-1相比Co)的0.85硒/钴9硒8(0.0102小号-1),Co的改进的催化活性的3 O 4 / Co 0.85 Se / Co 9 Se 8归因于存在更多的活动站点,而不是每个站点活动有所改善。C dl(双层电容)测量进一步支持了这一点,其中Co 3 O 4 / Co 0.85 Se / Co 9 Se 8和Co 0.85 Se / Co 9 Se 8的嫩C dl值约为8.19和1.08 mF / cm 2分别经过电化学预处理后。制备的Co 3 O 4 / Co 0.85 Se / Co 9 Se 8还表现出快速动力学(低Tafel斜度〜91 mV / dec),长期稳定性,低电荷转移电阻和碱性电催化(pH = 14)的法拉第效率为82%。此外,发现质子反应阶数(ρRHE)为0.65,表明碱性OER的质子解耦电子传递(PDET)机制。因此,Co 3 O 4载体有助于暴露更多的Co 0.85 Se / Co 9 Se 8催化位点,从而在碱性介质中提供改善的催化活性。
更新日期:2020-12-07
中文翻译:

Co 3 O 4作为微结构载体的引入,以促进Co 0.85 Se / Co 9 Se 8网络的碱性氧释放反应
开发具有丰富活性位点的电催化剂是降低碱性氧放出反应(OER)的过电位要求的一项重大挑战。在这项工作中,我们旨在通过使硒化钴在自支撑的Co 3 O 4微棒上生长来提高其催化活性。最初,通过对所制备的草酸钴前体进行退火来合成Co 3 O 4微棒。随后的Co 3 O 4硒化导致形成粒状棒状Co 3 O 4 / Co 0.85 Se / Co 9 Se 8网络。即使在硒化处理之后,硒化钴纳米颗粒被随机覆盖在Co 3 O 4载体上,结构和形态分析也显示出Co 3 O 4的存在。通过在330 mV的超电势下提供10 mA / cm 2 geo的基准电流密度,所得电极在碱性介质中对OER具有优异的电催化活性。作为比较,我们在相似的条件下开发了Co 0.85 Se / Co 9 Se 8并评估了其OER活性。该材料消耗360 mV的过电势以提供基准电流密度,这表明Co的作用3 O 4载体可改善Co 0.85 Se / Co 9 Se 8的电催化活性。尽管具有低TOF值钴3 ö 4 /钴0.85硒/钴9硒8(0.0076小号-1相比Co)的0.85硒/钴9硒8(0.0102小号-1),Co的改进的催化活性的3 O 4 / Co 0.85 Se / Co 9 Se 8归因于存在更多的活动站点,而不是每个站点活动有所改善。C dl(双层电容)测量进一步支持了这一点,其中Co 3 O 4 / Co 0.85 Se / Co 9 Se 8和Co 0.85 Se / Co 9 Se 8的嫩C dl值约为8.19和1.08 mF / cm 2分别经过电化学预处理后。制备的Co 3 O 4 / Co 0.85 Se / Co 9 Se 8还表现出快速动力学(低Tafel斜度〜91 mV / dec),长期稳定性,低电荷转移电阻和碱性电催化(pH = 14)的法拉第效率为82%。此外,发现质子反应阶数(ρRHE)为0.65,表明碱性OER的质子解耦电子传递(PDET)机制。因此,Co 3 O 4载体有助于暴露更多的Co 0.85 Se / Co 9 Se 8催化位点,从而在碱性介质中提供改善的催化活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号