当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Three-Way Switchable Single-Crystal-to-Single-Crystal Solvatomorphic Spin Crossover in a Molecular Cocrystal
Chemistry of Materials ( IF 7.2 ) Pub Date : 2020-11-20 , DOI: 10.1021/acs.chemmater.0c03396 Andrés Reyes Zuluaga 1, 2 , Aidan J. Brock 1, 2 , Michael C. Pfrunder 1, 2 , Wasinee Phonsri 3 , Keith S. Murray 3 , Phimphaka Harding 4 , Aaron S. Micallef 2, 5 , Kathleen M. Mullen 1, 2 , Jack K. Clegg 6 , David J. Harding 4 , John C. McMurtrie 1, 2
Chemistry of Materials ( IF 7.2 ) Pub Date : 2020-11-20 , DOI: 10.1021/acs.chemmater.0c03396 Andrés Reyes Zuluaga 1, 2 , Aidan J. Brock 1, 2 , Michael C. Pfrunder 1, 2 , Wasinee Phonsri 3 , Keith S. Murray 3 , Phimphaka Harding 4 , Aaron S. Micallef 2, 5 , Kathleen M. Mullen 1, 2 , Jack K. Clegg 6 , David J. Harding 4 , John C. McMurtrie 1, 2
Affiliation
|
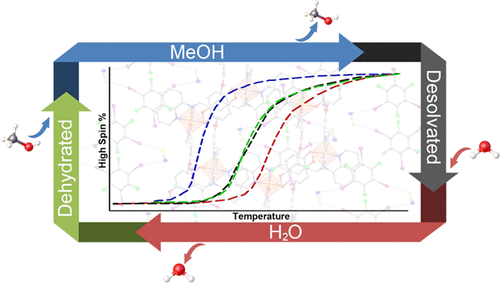
The Fe(III) spin-switching complexes [Fe(qsal-5-Y)2]X (where Y = F, Cl, Br, I or OMe; X = NCS–, Cl–, OTf–, NO3–, BF4–, PF6–, or BPh4–) display a variety of thermal spin transition profiles, including abrupt, stepped, and hysteretic, with potential applications as temperature- or gas-dependent switches or memory/storage devices. Here, the complex [Fe(qsal-OMe)2]NCS is encased within discrete anionic supramolecular motifs in cocrystalline [Fe(qsal-OMe)2][(1,3,5-triiodotrifluorobenzene)(NCS)]·MeOH/H2O (1·IFB·solvent). The MeOH and H2O solvate within these robust crystals can be reversibly exchanged, giving an artificial triple hysteresis, with six different stable magnetic states and a ΔT1/2 of 95 K (MeOH versus H2O solvatomorphs), which represent the workings of a stimulated logic gate that can be reset with heat or vacuum. Variable-temperature single-crystal X-ray diffraction (VT-SCXRD) elucidated the entire spin transition profile of the parent complex [Fe(qsal-OMe)2](NCS)·MeCN (1·MeCN) and the anionic supramolecular framework-like adducts [Fe(qsal-OMe)2][(1,3,5-triiodotrifluorobenzene)(NCS)] (1·IFB), [Fe(qsal-OMe)2][(1,3,5-triiodotrifluorobenzene)(NCS)]·MeOH (1·IFB·MeOH) and [Fe(qsal-OMe)2][(1,3,5-triiodotrifluorobenzene)(NCS)]·H2O (1·IFB·H2O), with full structures collected every 5 or 10 K (total of 202 individual structures) over a temperature range of 100–450 K. The reversible solvent exchange and magnetic changes suggest that crystalline 1·IFB may be used as a specialized molecular magnetic sensor for MeOH and H2O.
中文翻译:

分子共晶中的三向可切换单晶至单晶溶变自旋交叉
Fe(III)自旋转换复合物[Fe(qsal-5-Y)2 ] X(其中Y = F,Cl,Br,I或OMe; X = NCS –,Cl –,OTf –,NO 3 –, BF 4 –,PF 6 –或BPh 4 –)显示了多种热自旋转变曲线,包括突变,阶跃和滞后,具有潜在的应用,例如取决于温度或气体的开关或存储/存储设备。在这里,复合物[Fe(qsal-OMe)2 ] NCS被包裹在共晶[Fe(qsal-OMe)2 ] [(1,3,5-三碘三氟苯)(NCS)]·MeOH / H中的离散阴离子超分子基序内2 O(1 · IFB ·溶剂)。这些坚固的晶体中的MeOH和H 2 O溶剂化物可以可逆地交换,从而产生人工三重磁滞现象,具有六种不同的稳定磁态和95 K的ΔT 1/2(MeOH对H 2 O溶剂化物)。可以通过加热或真空复位的受激逻辑门的工作方式。可变温度单晶X射线衍射(VT-SCXRD)阐明了母体[Fe(qsal-OMe)2 ](NCS)·MeCN(1 · MeCN)和阴离子超分子骨架-像加合物[Fe(qsal-OMe)2] [((1,3,5-三碘三氟苯)(NCS)](1 · IFB),[Fe(qsal-OMe)2 ] [(1,3,5-三碘三氟苯)(NCS)]·甲醇(1 · IFB · MeOH)和[Fe(qsal-OMe)2 ] [(1,3,5-三碘三氟苯)(NCS)]·H 2 O(1 · IFB · H 2 O),每5或10 K收集一次完整结构(总共202个单独的结构)在100–450 K的温度范围内。可逆的溶剂交换和磁变化表明,结晶1 · IFB可以用作MeOH和H的专门分子磁性传感器2 O.
更新日期:2020-12-08
中文翻译:

分子共晶中的三向可切换单晶至单晶溶变自旋交叉
Fe(III)自旋转换复合物[Fe(qsal-5-Y)2 ] X(其中Y = F,Cl,Br,I或OMe; X = NCS –,Cl –,OTf –,NO 3 –, BF 4 –,PF 6 –或BPh 4 –)显示了多种热自旋转变曲线,包括突变,阶跃和滞后,具有潜在的应用,例如取决于温度或气体的开关或存储/存储设备。在这里,复合物[Fe(qsal-OMe)2 ] NCS被包裹在共晶[Fe(qsal-OMe)2 ] [(1,3,5-三碘三氟苯)(NCS)]·MeOH / H中的离散阴离子超分子基序内2 O(1 · IFB ·溶剂)。这些坚固的晶体中的MeOH和H 2 O溶剂化物可以可逆地交换,从而产生人工三重磁滞现象,具有六种不同的稳定磁态和95 K的ΔT 1/2(MeOH对H 2 O溶剂化物)。可以通过加热或真空复位的受激逻辑门的工作方式。可变温度单晶X射线衍射(VT-SCXRD)阐明了母体[Fe(qsal-OMe)2 ](NCS)·MeCN(1 · MeCN)和阴离子超分子骨架-像加合物[Fe(qsal-OMe)2] [((1,3,5-三碘三氟苯)(NCS)](1 · IFB),[Fe(qsal-OMe)2 ] [(1,3,5-三碘三氟苯)(NCS)]·甲醇(1 · IFB · MeOH)和[Fe(qsal-OMe)2 ] [(1,3,5-三碘三氟苯)(NCS)]·H 2 O(1 · IFB · H 2 O),每5或10 K收集一次完整结构(总共202个单独的结构)在100–450 K的温度范围内。可逆的溶剂交换和磁变化表明,结晶1 · IFB可以用作MeOH和H的专门分子磁性传感器2 O.









































 京公网安备 11010802027423号
京公网安备 11010802027423号