当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Interaction between Cerium and H2O in Hydrous Rhyolitic Melts
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2020-11-20 , DOI: 10.1021/acsearthspacechem.0c00206 Nozomi M. Kondo 1 , Yoshio Kono 1 , Koji Ohara 2 , Ryoichi Nakada 3 , Toshiaki Ina 2 , Etienne Skrzypek 4 , Akihiro Yamada 5 , Satoshi Saito 6
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2020-11-20 , DOI: 10.1021/acsearthspacechem.0c00206 Nozomi M. Kondo 1 , Yoshio Kono 1 , Koji Ohara 2 , Ryoichi Nakada 3 , Toshiaki Ina 2 , Etienne Skrzypek 4 , Akihiro Yamada 5 , Satoshi Saito 6
Affiliation
|
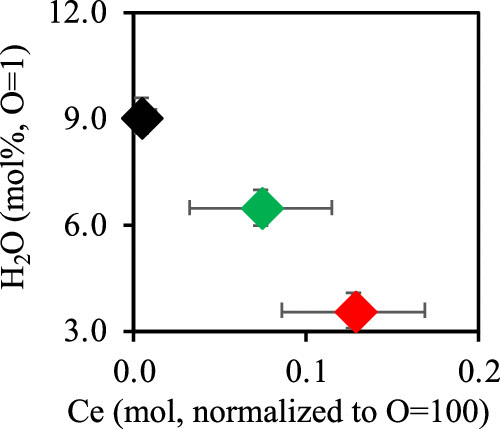
Cerium (Ce) in silicate melt is considered to provide important information about the nature of silicate magmas in the Earth’s and planetary interiors, especially about their oxidation state. However, the behavior of Ce in a silicate melt may not be simple, particularly in hydrous silicate melt, where a strong depression of the Ce4+/Ce3+ ratio occurs. Here we investigate the interaction between cerium and H2O in the structure of hydrous rhyolitic melts. Hydrous rhyolitic glasses quenched from melts at 1 GPa and 1300 °C show a significant decrease of H2O solubility by the incorporation of Ce. Pair distribution function analyses show no distinct change in the local structures of hydrous rhyolitic glasses with the incorporation of Ce, while we found a distinct change in the intensity of the T–OH (where T is Si or Al) Raman peak. The incorporation of Ce decreases the proportion of T–OH species and increases those of Q4 and Q3 species. These results suggest the occurrence of a direct charge transfer reaction between OH− in the melt and incorporated Ce as Cemelt4+ + 2(OH)melt– → Cemelt3+ + H2O + Omelt. This reaction causes a charge transfer from Ce4+ to Ce3+ in the hydrous rhyolitic melt, and it inevitably produces Ce3+. X-ray absorption near-edge structure (XANES) measurements show almost completely trivalent Ce (Ce3+) in the Ce-incorporated hydrous rhyolitic glasses, which supports the production of Ce3+ through the charge transfer reaction between Ce and OH−. The decrease of Ce4+/Ce3+ ratio reported in hydrous silicate melts would be due to the charge transfer reaction between Ce and H, which implies that the Ce4+/Ce3+ ratio in hydrous silicate melts may bear a complex relationship to the oxidation state of magmas.
中文翻译:

铈与H 2 O在水合流纹熔体中的相互作用
硅酸盐熔体中的铈(Ce)被认为可提供有关地球和行星内部硅酸盐岩浆性质的重要信息,尤其是有关其氧化态的信息。然而,Ce在硅酸盐熔体中的行为可能并不简单,特别是在含水的硅酸盐熔体中,在其中发生Ce 4+ / Ce 3+比的强烈降低。在这里,我们研究了铈与H 2 O在水合流纹岩熔体结构中的相互作用。在1 GPa和1300°C下从熔体中淬灭的水合流纹玻璃显示出H 2的显着降低通过掺入Ce来溶解O。配对分布函数分析表明,掺入Ce不会使含水流纹玻璃的局部结构发生明显变化,而我们发现T–OH(其中T为Si或Al)拉曼峰的强度发生明显变化。Ce的掺入减少了T-OH种类的比例,并增加了Q 4和Q 3种类的比例。这些结果表明OH之间的直接电荷转移反应的发生-在熔化并结合Ce作为Ce的熔融4+ + 2(OH)熔融- →Ce的熔体3+ + H 2 O +Ö熔体。该反应导致含水流纹岩熔体中的电荷从Ce 4+转移到Ce 3+,不可避免地产生Ce 3+。X射线吸收近边结构(XANES)的测量显示几乎完全的三价的Ce(铈3+)中的Ce-掺入含水流纹眼镜,它支持生产的Ce 3+通过Ce和之间OH电荷转移反应- 。含水硅酸盐熔体中报道的Ce 4+ / Ce 3+比值的降低是由于Ce和H之间的电荷转移反应,这意味着Ce 4+ / Ce 3+ 含水硅酸盐熔体中的比率可能与岩浆的氧化态具有复杂的关系。
更新日期:2020-12-17
中文翻译:

铈与H 2 O在水合流纹熔体中的相互作用
硅酸盐熔体中的铈(Ce)被认为可提供有关地球和行星内部硅酸盐岩浆性质的重要信息,尤其是有关其氧化态的信息。然而,Ce在硅酸盐熔体中的行为可能并不简单,特别是在含水的硅酸盐熔体中,在其中发生Ce 4+ / Ce 3+比的强烈降低。在这里,我们研究了铈与H 2 O在水合流纹岩熔体结构中的相互作用。在1 GPa和1300°C下从熔体中淬灭的水合流纹玻璃显示出H 2的显着降低通过掺入Ce来溶解O。配对分布函数分析表明,掺入Ce不会使含水流纹玻璃的局部结构发生明显变化,而我们发现T–OH(其中T为Si或Al)拉曼峰的强度发生明显变化。Ce的掺入减少了T-OH种类的比例,并增加了Q 4和Q 3种类的比例。这些结果表明OH之间的直接电荷转移反应的发生-在熔化并结合Ce作为Ce的熔融4+ + 2(OH)熔融- →Ce的熔体3+ + H 2 O +Ö熔体。该反应导致含水流纹岩熔体中的电荷从Ce 4+转移到Ce 3+,不可避免地产生Ce 3+。X射线吸收近边结构(XANES)的测量显示几乎完全的三价的Ce(铈3+)中的Ce-掺入含水流纹眼镜,它支持生产的Ce 3+通过Ce和之间OH电荷转移反应- 。含水硅酸盐熔体中报道的Ce 4+ / Ce 3+比值的降低是由于Ce和H之间的电荷转移反应,这意味着Ce 4+ / Ce 3+ 含水硅酸盐熔体中的比率可能与岩浆的氧化态具有复杂的关系。











































 京公网安备 11010802027423号
京公网安备 11010802027423号