当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Which Species, Zn2+ Cations or ZnO Clusters, Are More Efficient for Olefin Aromatization? 13C Solid-State NMR Investigation of n-But-1-ene Transformation on Zn-Modified Zeolite
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-11-20 , DOI: 10.1021/acscatal.0c03647 Zoya N. Lashchinskaya 1, 2 , Anton A. Gabrienko 1, 2 , Sergei S. Arzumanov 1, 2 , Alexander A. Kolganov 1 , Alexander V. Toktarev 1 , Dieter Freude 3 , Jürgen Haase 3 , Alexander G. Stepanov 1, 2
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-11-20 , DOI: 10.1021/acscatal.0c03647 Zoya N. Lashchinskaya 1, 2 , Anton A. Gabrienko 1, 2 , Sergei S. Arzumanov 1, 2 , Alexander A. Kolganov 1 , Alexander V. Toktarev 1 , Dieter Freude 3 , Jürgen Haase 3 , Alexander G. Stepanov 1, 2
Affiliation
|
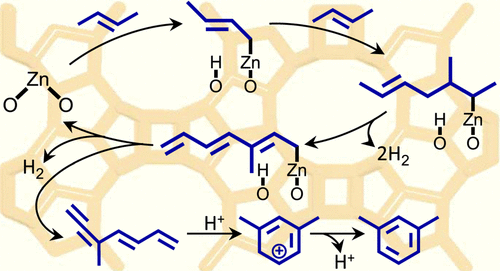
The analysis of n-but-1-ene transformation on Zn-modified zeolite H-BEA, containing zinc exclusively in the form of either Zn2+ cations (Zn2+/H-BEA sample) or small clusters of ZnO (ZnO/H-BEA sample), has been performed with 13C solid-state nuclear magnetic resonance (NMR) at 296–673 K. The number of intermediates, including π-complex of n-but-2-ene, methylallylzinc, and delocalized carbanionic species formed by the interaction of oligomeric polyenes with Zn sites, have been identified for both zeolite samples. Methyl-substituted cyclopentenyl cation and cyclohexadienyl cation are additionally identified for the reaction on ZnO/H-BEA. It is inferred that the aromatization of the olefin occurs basically with the involvement of Zn2+ sites on Zn2+/H-BEA. For ZnO/H-BEA, besides aromatization with the assistance of ZnO species, conjunct polymerization process with the involvement of Brønsted acid sites (BAS) contributes notably to the olefin aromatization. The latter process affords also some quantity of C1–C4 alkanes. It is concluded that the stronger interaction of the olefin (confirmed by density functional theory (DFT) calculations) and oligomeric polyenes with Zn2+ cations than with ZnO species and different quantities of BAS for two zeolite samples provide peculiar performances of Zn2+/H-BEA and ZnO/H-BEA zeolites for the olefin aromatization. Based on careful analysis of the obtained spectroscopic results, it is suggested that Zn-modified zeolite containing Zn2+ cationic species and some quantity of BAS should exhibit higher efficiency as the catalyst for small olefin and alkane aromatization compared to the zeolite with ZnO species and high concentration of BAS.
中文翻译:

哪种物种(Zn 2+阳离子或ZnO簇)对烯烃芳构化更有效?Zn改性沸石上正丁-1-烯转化的13 C固态NMR研究
Zn改性沸石H-BEA上正丁烯的转化分析,该沸石仅以Zn 2+阳离子的形式(锌2+ / H-BEA样品)或小的ZnO簇(ZnO / H-BEA样品),已在296–673 K的温度下进行了13 C固态核磁共振(NMR)。中间体的数量,包括n的π络合物,对于两种沸石样品,均已确定了由2-聚丁烯,低碳多烯与锌位点相互作用形成的1-丁-2-烯,甲基烯丙基锌和离域的碳负离子。另外鉴定了甲基取代的环戊烯基阳离子和环己二烯基阳离子用于在ZnO / H-BEA上的反应。据推断,烯烃的芳构化与锌的参与基本上发生2+对Zn网站2+ / H-BEA。对于ZnO / H-BEA,除了借助ZnO物种进行芳构化外,布朗斯台德酸位点(BAS)参与的混合聚合过程也显着促进了烯烃的芳构化。后一个过程还提供了一定数量的C 1 -C 4烷烃。结论是,对于两个沸石样品,烯烃(通过密度泛函理论(DFT)计算确认)与低聚多烯与ZnO物种和不同数量的BAS的较强相互作用比ZnO物种和Zn 2+阳离子的低相互作用提供了Zn 2+ / H-BEA和ZnO / H-BEA沸石用于烯烃芳构化。根据对所得光谱结果的仔细分析,建议与具有ZnO和ZnO的沸石相比,含有Zn 2+阳离子物质和一定数量的BAS的Zn改性沸石作为小烯烃和烷烃芳构化的催化剂应具有更高的效率。高浓度的BAS。
更新日期:2020-12-04
中文翻译:

哪种物种(Zn 2+阳离子或ZnO簇)对烯烃芳构化更有效?Zn改性沸石上正丁-1-烯转化的13 C固态NMR研究
Zn改性沸石H-BEA上正丁烯的转化分析,该沸石仅以Zn 2+阳离子的形式(锌2+ / H-BEA样品)或小的ZnO簇(ZnO / H-BEA样品),已在296–673 K的温度下进行了13 C固态核磁共振(NMR)。中间体的数量,包括n的π络合物,对于两种沸石样品,均已确定了由2-聚丁烯,低碳多烯与锌位点相互作用形成的1-丁-2-烯,甲基烯丙基锌和离域的碳负离子。另外鉴定了甲基取代的环戊烯基阳离子和环己二烯基阳离子用于在ZnO / H-BEA上的反应。据推断,烯烃的芳构化与锌的参与基本上发生2+对Zn网站2+ / H-BEA。对于ZnO / H-BEA,除了借助ZnO物种进行芳构化外,布朗斯台德酸位点(BAS)参与的混合聚合过程也显着促进了烯烃的芳构化。后一个过程还提供了一定数量的C 1 -C 4烷烃。结论是,对于两个沸石样品,烯烃(通过密度泛函理论(DFT)计算确认)与低聚多烯与ZnO物种和不同数量的BAS的较强相互作用比ZnO物种和Zn 2+阳离子的低相互作用提供了Zn 2+ / H-BEA和ZnO / H-BEA沸石用于烯烃芳构化。根据对所得光谱结果的仔细分析,建议与具有ZnO和ZnO的沸石相比,含有Zn 2+阳离子物质和一定数量的BAS的Zn改性沸石作为小烯烃和烷烃芳构化的催化剂应具有更高的效率。高浓度的BAS。











































 京公网安备 11010802027423号
京公网安备 11010802027423号