当前位置:
X-MOL 学术
›
ACS Appl. Bio Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of DNA Aptamer as a β-Amyloid Aggregation Inhibitor
ACS Applied Bio Materials ( IF 4.6 ) Pub Date : 2020-11-19 , DOI: 10.1021/acsabm.0c00996 Yan Zheng 1 , Pei Wang 1 , Shaoyuan Li 1 , Xiuhua Geng 1 , Liyuan Zou 1 , Meimei Jin 1 , Qingqing Zou 1 , Qing Wang 1 , Xiaohai Yang 1 , Kemin Wang 1
ACS Applied Bio Materials ( IF 4.6 ) Pub Date : 2020-11-19 , DOI: 10.1021/acsabm.0c00996 Yan Zheng 1 , Pei Wang 1 , Shaoyuan Li 1 , Xiuhua Geng 1 , Liyuan Zou 1 , Meimei Jin 1 , Qingqing Zou 1 , Qing Wang 1 , Xiaohai Yang 1 , Kemin Wang 1
Affiliation
|
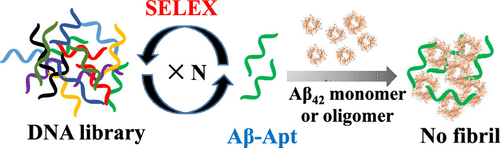
Developing a strategy of modulating β-amyloid (Aβ) aggregation with low cost, easy synthesis, high efficiency, and biosafety is significant and a challenge for Alzheimer’s disease (AD) therapy. Herein, DNA aptamer (Aβ-Apt) against Aβ42 obtained by in vitro selection was developed as a potent inhibitor of Aβ42 aggregation for the first time. Indeed, the Aβ42 monomer fibrillation was inhibited completely by Aβ-Apt. Notably, the inhibition effect of Aβ-Apt on the Aβ42 oligomer aggregation was more obvious than that on the Aβ42 monomer aggregation. It was presumed that the distinguishing effect may be attributed to different binding behaviors of Aβ-Apt with Aβ42 monomer and Aβ42 oligomer. Surface plasmon resonance analysis demonstrated that Aβ-Apt specifically recognized Aβ42 monomer and Aβ42 oligomer. Furthermore, the binding affinity of Aβ-Apt with Aβ42 oligomer was larger than that of Aβ-Apt with Aβ42 monomer. This work provided a promising platform with high efficiency for manipulating Aβ aggregation.
中文翻译:

DNA适体作为β-淀粉样蛋白聚集抑制剂的开发
开发一种低成本、易于合成、高效和生物安全的调节 β-淀粉样蛋白 (Aβ) 聚集的策略对于阿尔茨海默病 (AD) 治疗具有重要意义和挑战。在此,通过体外选择获得的针对 Aβ 42的 DNA 适体 (Aβ-Apt) 首次被开发为 Aβ 42聚集的有效抑制剂。实际上,Aβ-Apt 完全抑制了 Aβ 42单体原纤维化。值得注意的是,Aβ-Apt对Aβ 42寡聚体聚集的抑制作用比对Aβ 42单体聚集的抑制作用更明显。推测这种区分效应可能归因于 Aβ-Apt 与 Aβ 42单体和 Aβ的不同结合行为。42低聚物。表面等离子共振分析表明 Aβ-Apt 特异性识别 Aβ 42单体和 Aβ 42寡聚体。此外,Aβ-Apt 与 Aβ 42寡聚体的结合亲和力大于 Aβ-Apt 与 Aβ 42单体的结合亲和力。这项工作为操纵 Aβ 聚集提供了一个有前途的高效平台。
更新日期:2020-12-21
中文翻译:

DNA适体作为β-淀粉样蛋白聚集抑制剂的开发
开发一种低成本、易于合成、高效和生物安全的调节 β-淀粉样蛋白 (Aβ) 聚集的策略对于阿尔茨海默病 (AD) 治疗具有重要意义和挑战。在此,通过体外选择获得的针对 Aβ 42的 DNA 适体 (Aβ-Apt) 首次被开发为 Aβ 42聚集的有效抑制剂。实际上,Aβ-Apt 完全抑制了 Aβ 42单体原纤维化。值得注意的是,Aβ-Apt对Aβ 42寡聚体聚集的抑制作用比对Aβ 42单体聚集的抑制作用更明显。推测这种区分效应可能归因于 Aβ-Apt 与 Aβ 42单体和 Aβ的不同结合行为。42低聚物。表面等离子共振分析表明 Aβ-Apt 特异性识别 Aβ 42单体和 Aβ 42寡聚体。此外,Aβ-Apt 与 Aβ 42寡聚体的结合亲和力大于 Aβ-Apt 与 Aβ 42单体的结合亲和力。这项工作为操纵 Aβ 聚集提供了一个有前途的高效平台。











































 京公网安备 11010802027423号
京公网安备 11010802027423号