当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Diastereoselective Spirocyclization via Intramolecular C(sp3)−H Bond Functionalization Triggered by Sequential [1,5]‐Hydride Shift/Cyclization Process: Approach to Spiro‐tetrahydroquinolines
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-11-20 , DOI: 10.1002/adsc.202001011 Arup Bhowmik 1 , Sumit Das 1, 2 , Writhabrata Sarkar 1 , K. M. Saidalavi 1 , Aniket Mishra 1 , Anupama Roy 1 , Indubhusan Deb 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-11-20 , DOI: 10.1002/adsc.202001011 Arup Bhowmik 1 , Sumit Das 1, 2 , Writhabrata Sarkar 1 , K. M. Saidalavi 1 , Aniket Mishra 1 , Anupama Roy 1 , Indubhusan Deb 1
Affiliation
|
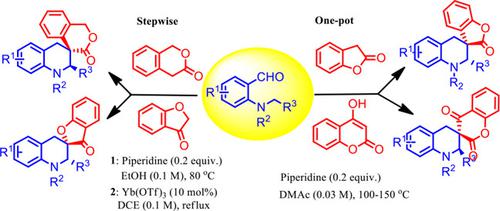
A direct synthesis of spiro[5.5]and [5.4]‐tetrahydroquinolines has been developed through C(sp3)−H bond functionalization triggered by sequential [1,5]‐ hydride shift /cyclization sequence using ortho amino benzaldehydes and active methylene compounds such as 2‐coumaranone, 4‐hydroxycoumarin, 3‐coumaranone, and 3‐isochromanone. This protocol provides a Lewis acid catalyst‐free straight forward one‐pot reaction in cases of 2‐coumaranone and 4‐hydroxycoumarin, Lewis acid‐catalyzed stepwise reaction for 3‐coumaranone and 3‐isochromanone to access a wide range of spiro‐heterocycles in excellent to good yields and diastereoselectivity.
中文翻译:

通过顺序[1,5]-氢化物转移/环化过程触发的分子内C(sp3)-H键功能非对映选择性螺环化:螺四氢喹啉的方法
通过邻氨基苯甲醛和活性亚甲基化合物等连续的[1,5]-氢化物转移/环化序列引发的C(sp 3)-H键官能化,开发了螺[5.5]和[5.4]-四氢喹啉的直接合成物作为2-香豆酮,4-羟基香豆素,3-香豆酮和3-异苯并二氢吡喃酮。该方案可在2-香豆香酮和4-羟基香豆素的情况下提供无Lewis酸的简单直接反应,而Lewis酸催化的3-香豆香酮和3-isochromanone的逐步反应可在其中使用广泛的螺杂环优异的良率和非对映选择性。
更新日期:2020-11-20
中文翻译:

通过顺序[1,5]-氢化物转移/环化过程触发的分子内C(sp3)-H键功能非对映选择性螺环化:螺四氢喹啉的方法
通过邻氨基苯甲醛和活性亚甲基化合物等连续的[1,5]-氢化物转移/环化序列引发的C(sp 3)-H键官能化,开发了螺[5.5]和[5.4]-四氢喹啉的直接合成物作为2-香豆酮,4-羟基香豆素,3-香豆酮和3-异苯并二氢吡喃酮。该方案可在2-香豆香酮和4-羟基香豆素的情况下提供无Lewis酸的简单直接反应,而Lewis酸催化的3-香豆香酮和3-isochromanone的逐步反应可在其中使用广泛的螺杂环优异的良率和非对映选择性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号