当前位置:
X-MOL 学术
›
Synth. Met.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bis(pyrrolidino)[60]fullerenes: promising photostable fullerene-based acceptors suppressing light-induced absorber degradation pathways
Synthetic Metals ( IF 4.0 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.synthmet.2020.116632 Alexander V. Mumyatov , Fedor A. Prudnov , Diana K. Sagdullina , Ilya V. Martynov , Liana N. Inasaridze , Alexander V. Chernyak , Andrey V. Maskaev , Ilya E. Kuznetsov , Alexander V. Akkuratov , Pavel A. Troshin
Synthetic Metals ( IF 4.0 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.synthmet.2020.116632 Alexander V. Mumyatov , Fedor A. Prudnov , Diana K. Sagdullina , Ilya V. Martynov , Liana N. Inasaridze , Alexander V. Chernyak , Andrey V. Maskaev , Ilya E. Kuznetsov , Alexander V. Akkuratov , Pavel A. Troshin
|
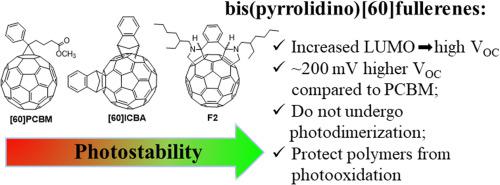
Abstract Two novel highly soluble bis(pyrrolidino)[60]fullerenes were synthesized and investigated as promising electron acceptor materials for organic solar cells. Reduced electron affinity of bis(pyrrolidino)[60]fullerenes as compared to the reference [60]PCBM enabled a spectacular increase in open-circuit voltage of organic bulk heterojunction solar cells by ~200 mV while using model P3HT and PCDTBT electron donor polymers. In addition to the improved optoelectronic properties, bis(pyrrolidino)[60]fullerenes were found to be resistant towards the photochemical cross-linking in contrast to conventional [60]PCBM and conjugated polymers. Most importantly, the designed fullerene derivatives efficiently suppressed photooxidation of conjugated polymers as revealed by ESR spectroscopy, which features a tremendous potential of applying these materials in the development of efficient and durable organic photovoltaics.
中文翻译:

双(吡咯烷)[60] 富勒烯:有前途的基于富勒烯的光稳定受体抑制光诱导吸收剂降解途径
摘要 合成并研究了两种新型的高溶解性双(吡咯烷)[60]富勒烯作为有机太阳能电池的电子受体材料。与参考文献 [60]PCBM 相比,双(吡咯烷)[60] 富勒烯的电子亲和性降低,使有机本体异质结太阳能电池的开路电压显着增加约 200 mV,同时使用模型 P3HT 和 PCDTBT 电子供体聚合物。除了改进的光电性能外,与传统的 [60] PCBM 和共轭聚合物相比,双(吡咯烷基)[60] 富勒烯被发现对光化学交联具有抵抗力。最重要的是,ESR 光谱显示,设计的富勒烯衍生物有效抑制了共轭聚合物的光氧化,
更新日期:2021-01-01
中文翻译:

双(吡咯烷)[60] 富勒烯:有前途的基于富勒烯的光稳定受体抑制光诱导吸收剂降解途径
摘要 合成并研究了两种新型的高溶解性双(吡咯烷)[60]富勒烯作为有机太阳能电池的电子受体材料。与参考文献 [60]PCBM 相比,双(吡咯烷)[60] 富勒烯的电子亲和性降低,使有机本体异质结太阳能电池的开路电压显着增加约 200 mV,同时使用模型 P3HT 和 PCDTBT 电子供体聚合物。除了改进的光电性能外,与传统的 [60] PCBM 和共轭聚合物相比,双(吡咯烷基)[60] 富勒烯被发现对光化学交联具有抵抗力。最重要的是,ESR 光谱显示,设计的富勒烯衍生物有效抑制了共轭聚合物的光氧化,









































 京公网安备 11010802027423号
京公网安备 11010802027423号