Journal of Rare Earths ( IF 5.2 ) Pub Date : 2020-11-21 , DOI: 10.1016/j.jre.2020.11.011 Jian Li , Yingjie Shi , Xiaoheng Fu , Yun Shu , Jiayu Huang , Jinwei Zhu , Gang Tian , Jingnan Hu
|
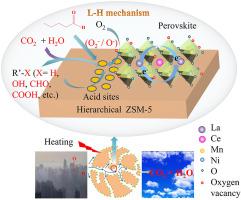
Hierarchical ZSM-5 (HZ) molecular sieves based on fly ash were synthesized using a method combining water heat treatment with step-by-step calcination. The coupling catalysts between La1−xCexMn0.8Ni0.2O3 (x ≤ 0.5) perovskites and HZ were prepared through the impregnation method, which were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), high-resolution transmission electron microscopy (HRTEM), N2 adsorption, X-ray photoelectron spectroscopy (XPS), NH3-temperature programmed desoprtion (NH3-TPD), H2-temperature programmed reduction (H2-TPR) and O2-TPD techniques and investigated regarding pentanal oxidation at 120−390 °C to explore the effects of Ce doping on the catalytic activity and the active oxygen species of the coupling catalysts, meanwhile, the reaction mechanism and pathway of pentanal oxidation were also studied. The results reveal that Ce substitution at La sites can change the electronic interactions between all the elements and promote the electronic transfer among La, Ce, Ni, Mn and HZ, influencing directly the physicochemical characteristics of the catalysts. Moreover, the amount and transfer ability of surface adsorbed oxygen (O2– and O–) regarded as the reactive oxygen species and the low temperature reducibility are the main influence factors in pentanal oxidation. Additionally, La0.8Ce0.2Mn0.8Ni0.2O3/HZ exhibits the best catalytic activity and deep oxidation capacity as well as a better water resistance due to its larger amount of surface adsorbed oxygen species and higher low temperature reducibility. What’s more, appropriate Ce substitution can significantly enhance the amount of O2– ions, which can distinctly enhance the catalytic activity of the catalyst, and moderate acid strength and appropriate acid amount can also facilitate the improvement of the pentanal oxidation activity. It is found that there is a synergic catalytic effect between surface acidity and redox ability of the catalyst. According to the in situ DRIFTS and GC/MS analyses, pentanal can be oxidized gradually to CO2 and H2O by the surface oxygen species with the form of adsorption in air following the Langmuir–Hinshelwood (L-H) reaction mechanism. Two reaction pathways for the pentanal oxidation process are proposed, and the conversion of the formates to carbonates may be one of the main rate-determining steps.
中文翻译:

Ce掺杂的LaMn 0.8 Ni 0.2 O 3 /分级ZSM-5在戊醛氧化中的活性氧物种和氧化机制
使用水热处理和分步煅烧相结合的方法合成了基于粉煤灰的分级 ZSM-5 (HZ) 分子筛。采用 浸渍法制备了La 1− x Ce x Mn 0.8 Ni 0.2 O 3 ( x ≤ 0.5) 钙钛矿与 HZ偶联催化剂,并通过 X 射线衍射 (XRD)、扫描电子显微镜 (SEM)、高分辨率透射电子显微镜 (HRTEM)、N 2吸附、X 射线光电子能谱 (XPS)、NH 3 -程序升温脱附 (NH 3 -TPD)、H 2 -程序升温还原 (H2 -TPR) 和 O 2 -TPD 技术,并研究了 120-390 °C 下戊醛氧化,以探讨 Ce 掺杂对偶联催化剂的催化活性和活性氧物种的影响,同时,反应机理和途径戊醛氧化也进行了研究。结果表明,La位点的Ce取代可以改变所有元素之间的电子相互作用,促进La、Ce、Ni、Mn和HZ之间的电子转移,直接影响催化剂的理化特性。此外,表面吸附氧(O 2 -和O -) 被认为是活性氧和低温还原性是戊醛氧化的主要影响因素。此外,La 0.8 Ce 0.2 Mn 0.8 Ni 0.2 O 3 /HZ 表现出最好的催化活性和深度氧化能力以及更好的耐水性,这是由于其表面吸附的氧种类较多,低温还原性较高。更重要的是,适当的Ce取代可以显着提高O 2 –离子,可以明显提高催化剂的催化活性,适度的酸强度和适当的酸量也有利于戊醛氧化活性的提高。发现催化剂的表面酸性和氧化还原能力之间存在协同催化作用。根据原位DRIFTS 和 GC/MS 分析,戊醛可以根据Langmuir-Hinshelwood (LH) 反应机制,通过空气中的吸附形式被表面氧物质逐渐氧化为 CO 2和 H 2 O。提出了戊醛氧化过程的两种反应途径,甲酸盐向碳酸盐的转化可能是主要的速率决定步骤之一。











































 京公网安备 11010802027423号
京公网安备 11010802027423号