当前位置:
X-MOL 学术
›
Gas Sci. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of Temperature on the Electrochemical Pitting Corrosion Behavior of 316L Stainless Steel in Chloride-Containing MDEA Solution
Gas Science and Engineering Pub Date : 2021-02-01 , DOI: 10.1016/j.jngse.2020.103718 Xianglong Hou , Quanyou Ren , Youkun Yang , Xianlong Cao , Jie Hu , Cheng Zhang , Hongda Deng , Daliang Yu , Kejian Li , Wei Lan
Gas Science and Engineering Pub Date : 2021-02-01 , DOI: 10.1016/j.jngse.2020.103718 Xianglong Hou , Quanyou Ren , Youkun Yang , Xianlong Cao , Jie Hu , Cheng Zhang , Hongda Deng , Daliang Yu , Kejian Li , Wei Lan
|
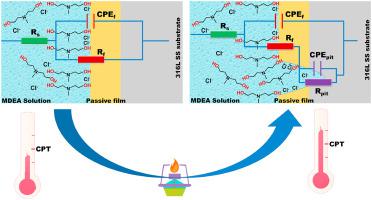
Abstract Here, the effect of temperature on the electrochemical corrosion of 316L stainless steel (SS) in methyl diethanolamine (MDEA) solution with/without 1.0 wt.% NaCl in the presence/absence of CO2 was investigated using potentiostatic polarization, potentiodynamic polarization, and electrochemical impedance spectroscopy (EIS). The electrochemical measurements showed that although the MDEA solution was highly basic, both chloride and temperature played greater roles in the pitting corrosion of 316L SS, similar to plain NaCl solution. The critical pitting temperature (CPT) of 316L SS in MDEA solution with 1.0 wt.% NaCl decreased from 67.2 oC to 59.6 oC, and the breakdown potential underwent a negative shift of approximately 400 mV, and the EIS Bode impedance decreased. Furthermore, the breakdown potential shifted negatively with increasing temperature when the temperature was lower than CPT in the MDEA solution containing Cl−; however, the breakdown potential decreased slowly, and its temperature dependence was unclear when the temperature was higher than the CPT. In addition, the passive film resistance of 316L SS in all MDEA solutions decreased as the temperature increased. Particularly for the MDEA solution containing Cl−, the passive film resistance decreased by one order of magnitude when the temperature increased from 50 °C to 60 °C. Although the presence of CO2 in the MDEA solution decreased the passive film resistance, it seemed to reduce the individual corrosion contribution of Cl−. The pitting corrosion process of 316L SS in MDEA solution containing chloride ions was discussed by combining these results with classical pitting mechanisms of stainless steel.
中文翻译:

温度对316L不锈钢在含氯MDEA溶液中电化学点蚀行为的影响
摘要 在此,使用恒电位极化、动电位极化和不存在 CO2 的情况下,温度对 316L 不锈钢 (SS) 在含/不含 1.0 wt.% NaCl 的甲基二乙醇胺 (MDEA) 溶液中电化学腐蚀的影响进行了研究。电化学阻抗谱 (EIS)。电化学测量表明,尽管 MDEA 溶液是强碱性的,但氯化物和温度在 316L SS 的点蚀中起着更大的作用,类似于普通的 NaCl 溶液。316L SS 在含 1.0 wt.% NaCl 的 MDEA 溶液中的临界点蚀温度 (CPT) 从 67.2 oC 降低到 59.6 oC,击穿电位负移约 400 mV,EIS 波德阻抗降低。此外,在含Cl−的MDEA溶液中,当温度低于CPT时,击穿电位随温度升高而负移;然而,击穿电位下降缓慢,当温度高于 CPT 时,其温度依赖性尚不清楚。此外,316L SS 在所有 MDEA 溶液中的钝化膜电阻随着温度的升高而降低。特别是对于含有 Cl− 的 MDEA 溶液,当温度从 50 °C 升高到 60 °C 时,钝化膜电阻降低一个数量级。尽管 MDEA 溶液中 CO2 的存在降低了钝化膜电阻,但它似乎降低了 Cl- 的个别腐蚀贡献。
更新日期:2021-02-01
中文翻译:

温度对316L不锈钢在含氯MDEA溶液中电化学点蚀行为的影响
摘要 在此,使用恒电位极化、动电位极化和不存在 CO2 的情况下,温度对 316L 不锈钢 (SS) 在含/不含 1.0 wt.% NaCl 的甲基二乙醇胺 (MDEA) 溶液中电化学腐蚀的影响进行了研究。电化学阻抗谱 (EIS)。电化学测量表明,尽管 MDEA 溶液是强碱性的,但氯化物和温度在 316L SS 的点蚀中起着更大的作用,类似于普通的 NaCl 溶液。316L SS 在含 1.0 wt.% NaCl 的 MDEA 溶液中的临界点蚀温度 (CPT) 从 67.2 oC 降低到 59.6 oC,击穿电位负移约 400 mV,EIS 波德阻抗降低。此外,在含Cl−的MDEA溶液中,当温度低于CPT时,击穿电位随温度升高而负移;然而,击穿电位下降缓慢,当温度高于 CPT 时,其温度依赖性尚不清楚。此外,316L SS 在所有 MDEA 溶液中的钝化膜电阻随着温度的升高而降低。特别是对于含有 Cl− 的 MDEA 溶液,当温度从 50 °C 升高到 60 °C 时,钝化膜电阻降低一个数量级。尽管 MDEA 溶液中 CO2 的存在降低了钝化膜电阻,但它似乎降低了 Cl- 的个别腐蚀贡献。











































 京公网安备 11010802027423号
京公网安备 11010802027423号