Journal of Inorganic Biochemistry ( IF 3.8 ) Pub Date : 2020-11-21 , DOI: 10.1016/j.jinorgbio.2020.111314 Mariana P Cali 1 , Lorena M B Pereira 1 , Marcio D Teodoro 2 , Tarciso A Sellani 3 , Elaine G Rodrigues 3 , Rose M Carlos 1
|
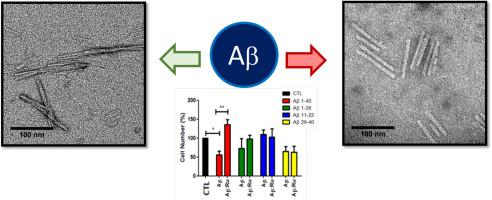
Neurotoxicity of amyloid beta (Aβ) species generated in early stages of aggregation has been associated with development of Alzheimer's disease (AD). Consequently, the field of action of compounds that can identify and inhibit the formation of these species has enlarged considerably. This study investigates the effect and influence of the luminescent, water soluble metal complex cis-[Ru(phen)2(3,4Apy)2]2+ (RuApy, 3,4Apy = 3,4-diaminopyridine, phen = 1,10-phenanthroline) on the aggregation process and toxicity of Aβ1–40 and its Aβ1–28, Aβ11–22 and Aβ29–40 fragments since their early stages. The absence of correlation between the conformations generated by Aβ fragments and the full length 1–40 peptide during aggregation and the absence of toxicity of Aβ fragments to PC12 cells in all stages of aggregation indicated that the aggregation pathway and toxicity found to the full-length Aβ1–40 depends on specific interactions between the three fragments. The toxicity of Aβ1–40 was dependent on the aggregation step investigated: species generated at the beginning (15 min) of aggregation were toxic, whereas mature (120 min) fibrils were not. The RuApy complex is not toxic to PC12 cells up to 60 μM, and does not interfere with the aggregation pathway of the Aβ fragments, but interferes with the aggregation of Aβ1–40 and protects the PC12 cells, maintaining 100% of cell viability against the toxicity of Aβ1–40 species generated in early stages of aggregation.
中文翻译:

Aβ(1–40、1–28、11–22 和 29–40)聚集过程的比较以及水溶性钌络合物对聚集早期产生的有毒物质的抑制
在聚集的早期阶段产生的淀粉样蛋白 β (Aβ) 的神经毒性与阿尔茨海默病 (AD) 的发展有关。因此,可以识别和抑制这些物种形成的化合物的作用领域已大大扩大。本研究调查了发光的水溶性金属络合物顺式-[Ru(phen) 2 (3,4Apy) 2 ] 2+ (RuApy, 3,4Apy = 3,4-diaminopyridine, phen = 1,10 -菲咯啉)对 Aβ 1-40及其 Aβ 1-28、Aβ 11-22和 Aβ 29-40的聚集过程和毒性的影响从早期阶段开始的碎片。聚集过程中 Aβ 片段产生的构象与全长 1-40 肽之间没有相关性,并且 Aβ 片段在聚集的各个阶段对 PC12 细胞没有毒性表明聚集途径和毒性发现到全长Aβ 1-40取决于三个片段之间的特定相互作用。Aβ 1-40的毒性取决于所研究的聚合步骤:在聚合开始(15 分钟)时产生的物种是有毒的,而成熟(120 分钟)的原纤维则没有。RuApy 复合物对高达 60 μM 的 PC12 细胞无毒,不会干扰 Aβ 片段的聚集途径,但会干扰 Aβ 1-40的聚集并保护 PC12 细胞,保持 100% 的细胞活力,抵抗在聚集早期产生的 Aβ 1-40物种的毒性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号