Journal of Hazardous Materials ( IF 13.6 ) Pub Date : 2020-11-20 , DOI: 10.1016/j.jhazmat.2020.124629 Ting Chen , Zhiliang Zhu , Hua Zhang , Yanling Qiu , Daqiang Yin
|
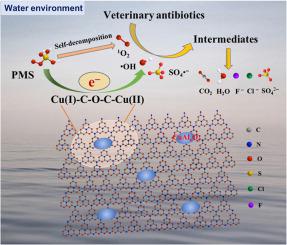
Constructing metal-oxo-bridge coordination as electron transfer bridge is benefit for the catalysis acceleration towards water pollutants treatment. In this work, regulable Cu–O–C configuration was constructed through tunable ratio of copper aluminum oxide (CuAlxOy) and porous g-C3N4 (p-CN), employed as electron transfer bridge for Cu(II)/Cu(I) redox behavior modulation to realize sufficient peroxymonosulfate (PMS) activation. A series of characterization demonstrated that the composites presents porous structure for p-CN contribution, and the oxygen content plays crucial role in Cu–O–C bonds fabrication. The proper ratio of CuAlxOy and p-CN is conducive to create an oxygen-rich environment, resulting in high dispersed copper atoms connect with oxygen in p-CN matrix. As a result, 2:1 CuAl@p-CN possesses appropriate Cu–O–C configuration, has favorable electron transfer environment for rapid redox behavior of Cu(II)/Cu(I), leading to excellent PMS activation for mirco-polluted veterinary antibiotics elimination. The activated PMS produced active species and followed the priority order of 1O2 > SO4•− > •OH for contaminants degradation, and the specific porous structure reduce the migration distance of active species for efficient catalysis. This study offers a deeper insight into the construction of regulable metal-oxo-bridge configurations for electron transfer towards PMS activation and contributes to an efficient strategy for wastewater remediation.
中文翻译:

可调节的金属-氧桥结构作为电子转移桥,可促进Cu(II)/ Cu(I)的氧化还原行为,从而有效地活化过氧一硫酸盐
将金属-氧-桥键配成电子传递桥有利于加速对水污染物的催化处理。在这项工作中,通过可调节比例的铜铝氧化物(CuAl x O y)和多孔gC 3 N 4(p-CN)来构造可调节的Cu-O-C结构,用作铜(II) / Cu的电子传递桥(I)氧化还原行为调节,以实现足够的过氧单硫酸盐(PMS)活化。一系列表征表明,复合材料呈现出对p-CN贡献的多孔结构,并且氧含量在Cu-O-C键的制造中起着至关重要的作用。CuAl x O y的适当比例p-CN有利于创造一个富氧的环境,导致高分散的铜原子与p-CN基质中的氧连接。因此,2:1 CuAl @ p-CN具有适当的Cu–O–C构型,具有良好的电子传递环境,可实现Cu (II) / Cu (I)的快速氧化还原行为,从而导致对微污染的PMS具有出色的活化作用消除兽用抗生素。激活的PMS产生了活性物质,并按照1 O 2 > SO 4 • −的优先顺序排列。>•OH,可降解污染物,特定的多孔结构可减少活性物质的迁移距离,从而实现高效催化。这项研究为可调节的金属-氧桥结构的构建提供了更深入的见解,以实现电子向PMS活化的转移,并为废水修复的有效策略做出了贡献。



























 京公网安备 11010802027423号
京公网安备 11010802027423号