Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2020-11-20 , DOI: 10.1016/j.jhazmat.2020.124616 Hugang Li , Maojiong Cao , Jamison Watson , Yuanhui Zhang , Zhidan Liu
|
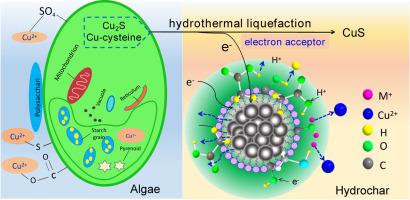
Cu is one of the dominant heavy metals toxic to human health and environmental ecosystems. Understanding its fate and chemical speciation is of great importance for hydrothermal liquefaction (HTL) of Cu-rich hazardous streams. Herein, we investigated its evolution during the HTL of wastewater algae through ICP-MS, XRD, XANES, and EXAFS. Cu-cysteine complexes (51.5%) and Cu2S (40.4%) were the main components of Cu in algae, whereas the predominant form was CuS (70.9%) in 220oC-hydrochar. Model compound experiments indicated that Cu-cysteine could be converted into CuS, while Cu2S was stable during HTL. However, Cu2S was partially converted into CuS in the hydrochar. Subsequently, the positive Gibbs free energy (36.8KJ/mol) indicates that the oxidation from Cu+ to Cu2+ can’t occur spontaneously. Furthermore, cyclic voltammograms demonstrated that hydrochar facilitated the oxidation of Cu2S due to its higher capability of electron acceptance. All these results prove that hydrochar serves as a catalyst for the conversion of Cu2S to CuS during HTL. This study firstly elucidated that Cu2S was oxidized into CuS in the presence of hydrochar, and Cu-cysteine was converted into CuS under HTL. This study provides a critical insight into the transformation mechanism of Cu during the HTL of hazardous streams.
中文翻译:

原位水焦调节铜的命运和形态:转化机制的见解。
铜是对人体健康和环境生态系统有毒的主要重金属之一。了解其命运和化学形态对于富含铜的危险物流的水热液化(HTL)至关重要。在这里,我们通过ICP-MS,XRD,XANES和EXAFS研究了废水藻类HTL期间其演变。铜-半胱氨酸配合物(51.5%)和铜2 S(40.4%)是藻类中铜的主要成分,而主要形式是在220 o C的碳氢盐中的铜(CuS)(70.9%)。模型化合物实验表明,Cu半胱氨酸可转化为CuS,而HTL期间Cu 2 S稳定。但是,Cu 2S在水焦中部分转化为CuS。随后,正吉布斯自由能(36.8KJ / mol)表明不能自发发生从Cu +到Cu 2+的氧化。此外,循环伏安图表明,水煤由于其较高的电子接受能力而促进了Cu 2 S的氧化。所有这些结果证明,在HTL期间,水焦炭可作为Cu 2 S转化为CuS的催化剂。该研究首先阐明了在水煤油存在下Cu 2 S被氧化为CuS,而HTL催化下Cu-半胱氨酸被转化为CuS。这项研究提供了对有害流HTL过程中Cu转化机理的重要见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号