当前位置:
X-MOL 学术
›
Fluid Phase Equilibr.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Raoult was Right After All: Statistical Mechanics Derivation and Volumetric Validation
Fluid Phase Equilibria ( IF 2.8 ) Pub Date : 2021-03-01 , DOI: 10.1016/j.fluid.2020.112899 Anthony S. Wexler
Fluid Phase Equilibria ( IF 2.8 ) Pub Date : 2021-03-01 , DOI: 10.1016/j.fluid.2020.112899 Anthony S. Wexler
|
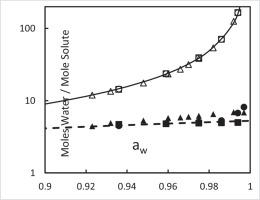
Abstract In prior work, we assumed that solutes hydrate and that water and the hydrated solutes form an ideal solution. These assumptions led to closed form solutions for both molality and solute activity as a function of water activity. Concentration-activity data of a wide range of solutes were accurately fit by the model. In this work we advance the model by (a) using statistical mechanics to derive the Gibbs free energy of the solute-water system and (b) applying the model to data for a number of sugars. The results show that the number of water molecules hydrogen bonded to the sugars is equal to the number of oxygen atoms in the sugar and that one free energy value, presumably the net hydrogen bond free energy, fits the concentration-activity data pretty well. The model also predicts the number of water molecules bound to the solute and the number of water molecules that are free. Hydration number as a function of water activity from the literature were compared to model predictions finding remarkable agreement.
中文翻译:

Raoult 是对的:统计力学推导和体积验证
摘要 在之前的工作中,我们假设溶质水合,并且水和水合溶质形成理想的溶液。这些假设导致摩尔浓度和溶质活性作为水分活度的函数的封闭形式解。该模型准确拟合了各种溶质的浓度-活性数据。在这项工作中,我们通过 (a) 使用统计力学推导出溶质-水系统的吉布斯自由能和 (b) 将模型应用于许多糖的数据来推进模型。结果表明,与糖键合的水分子的数量等于糖中氧原子的数量,并且一个自由能值,大概是净氢键自由能,非常符合浓度-活性数据。该模型还预测了与溶质结合的水分子的数量和游离的水分子的数量。将文献中作为水分活度函数的水合数与模型预测进行了比较,发现了显着的一致性。
更新日期:2021-03-01
中文翻译:

Raoult 是对的:统计力学推导和体积验证
摘要 在之前的工作中,我们假设溶质水合,并且水和水合溶质形成理想的溶液。这些假设导致摩尔浓度和溶质活性作为水分活度的函数的封闭形式解。该模型准确拟合了各种溶质的浓度-活性数据。在这项工作中,我们通过 (a) 使用统计力学推导出溶质-水系统的吉布斯自由能和 (b) 将模型应用于许多糖的数据来推进模型。结果表明,与糖键合的水分子的数量等于糖中氧原子的数量,并且一个自由能值,大概是净氢键自由能,非常符合浓度-活性数据。该模型还预测了与溶质结合的水分子的数量和游离的水分子的数量。将文献中作为水分活度函数的水合数与模型预测进行了比较,发现了显着的一致性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号