Chem ( IF 23.5 ) Pub Date : 2020-11-20 , DOI: 10.1016/j.chempr.2020.10.026 Wei Wang , Jun Wu , Rositha Kuniyil , Adelina Kopp , Rafaely Nascimento Lima , Lutz Ackermann
|
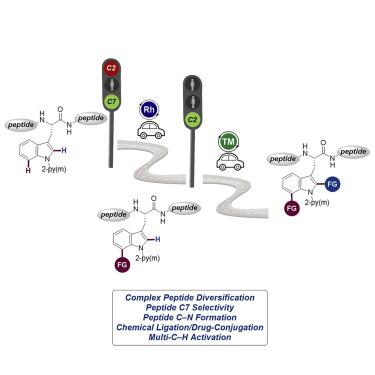
The late-stage diversification (LSD) of structurally complex peptides has emerged as a powerful platform for molecular engineering and drug discovery. Besides traditional peptide modifications, such as native chemical ligations or cross-couplings with prefunctionalized substrates, during recent years, C―H activation has gained considerable momentum as a robust and step-economical strategy for late-stage peptide modifications, thus far predominantly for the formation of C―C bonds. Although C―N bond formations represent established strategies in medicinal chemistry and drug discovery, methods for direct amidations of tryptophan and tryptophan-containing peptides are rare and severely limited to the activated C2 position. In contrast, we herein disclose the first example of direct late-stage peptide C―H amidation reaction and the unprecedented late-stage peptide diversification on tryptophan C7 position in a highly site-selective manner. Moreover, this strategy sets the stage for sequential double C(7)―H/C(2)―H modifications, further improving the peptide structural complexity.
中文翻译:

铑催化的色氨酸C7酰胺化的肽后期多样化
结构复杂肽的后期分散化(LSD)已经成为分子工程和药物发现的强大平台。除了传统的肽修饰(例如天然化学连接或与预功能化底物的交叉偶联)外,近年来,C-H活化作为后期肽修饰的一种稳健而经济的策略获得了可观的发展势头,迄今为止主要用于形成CC键。尽管C–N键的形成代表了药物化学和药物发现中的既定策略,但是色氨酸和含色氨酸的肽直接酰胺化的方法很少,并且严重限于活化的C2位置。相反,我们在本文中公开了直接后期肽CH酰胺化反应的第一个实例,以及以高度位点选择性的方式在色氨酸C7位置上空前的后期肽多样化。而且,该策略为连续双C(7)-H / C(2)-H修饰奠定了基础,进一步提高了肽的结构复杂性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号