Archives of Biochemistry and Biophysics ( IF 3.8 ) Pub Date : 2020-11-21 , DOI: 10.1016/j.abb.2020.108688 So Young Kim , Hyun Hwangbo , Min Yeong Kim , Seon Yeong Ji , Hyesook Lee , Gi-Young Kim , Chan-Young Kwon , Sun-Hee Leem , Su Hyun Hong , JaeHun Cheong , Yung Hyun Choi
|
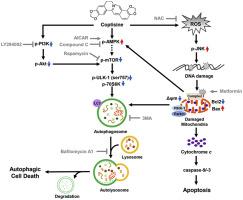
Coptisine is isoquinoline alkaloid derived from Coptidis Rhizoma and is known to have potential anti-cancer activity toward various carcinomas. Targeting autophagy is one of the main approaches for cancer therapy, but whether the anti-cancer efficacy of coptisine involves autophagy is still unclear. Therefore, this study investigated the effect of coptisine on autophagy in hepatocellular carcinoma (HCC) Hep3B cells, and identified the underlying mechanism. Our results showed that coptisine increased cytotoxicity and autophagic vacuoles in a concentration-dependent manner. Furthermore, the expressions of light chain 3 (LC3)-I/II, Beclin-1 and autophagy genes were markedly increased by coptisine, while the expression of p62 decreased. In addition, we found that pretreatment with bafilomycin A1, an inhibitor of autophagosome-lysosome fusion, markedly reduced coptisine-mediated autophagic cell death, but 3-methyladenine, an inhibitor for autophagosome formation did not. Moreover, our results showed that although coptisine up-regulated AMP-activated protein kinase (AMPK) that partially induced LC3-I/II, coptisine-mediated AMPK signaling did not directly regulate autophagic cell death. Additionally, we found that coptisine suppressed the phosphorylation of phosphoinositide 3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/Akt/mTOR), and this effect was notably enhanced by PI3K inhibitor LY294002. Meanwhile, coptisine significantly increased both the production of mitochondrial reactive oxygen species (ROS) and the recruitment of mitophagy-regulated proteins to mitochondria. Furthermore, N‐acetylcysteine, a potential ROS scavenger, substantially suppressed the expression of mitophagy-regulated proteins and LC3 puncta by coptisine. Overall, our results demonstrate that coptisine-mediated autophagic cell death was regulated by PI3K/Akt/mTOR signaling and mitochondrial ROS production associated with mitochondrial dysfunction. Taken together, these findings suggest that coptisine exerts its anti-cancer effects through induction of autophagy in HCC Hep3B cells.
中文翻译:

黄连素通过下调PI3K / Akt / mTOR信号通路和上调ROS介导的肝细胞癌Hep3B细胞线粒体功能障碍来诱导自噬细胞死亡
黄连碱是衍生自黄连的异喹啉生物碱,已知对多种癌具有潜在的抗癌活性。靶向自噬是癌症治疗的主要方法之一,但黄连的抗癌功效是否涉及自噬仍不清楚。因此,本研究调查了黄连碱对肝细胞癌(HCC)Hep3B细胞自噬的影响,并确定了其潜在机制。我们的结果表明,黄连以浓度依赖的方式增加了细胞毒性和自噬空泡。此外,黄连碱显着增加了轻链3(LC3)-I / II,Beclin-1和自噬基因的表达,而p62的表达却降低了。此外,我们发现用bafilomycin A1(自噬小体-溶酶体融合抑制剂)进行预处理,显着减少了黄连介导的自噬细胞死亡,但自噬体形成抑制剂3-甲基腺嘌呤却没有。此外,我们的结果表明,尽管黄连上调了部分诱导LC3-I / II的AMP活化蛋白激酶(AMPK),但黄连介导的AMPK信号并未直接调节自噬细胞的死亡。此外,我们发现黄连抑制磷酸肌醇3-激酶/蛋白激酶B /雷帕霉素的哺乳动物靶标(PI3K / Akt / mTOR)的磷酸化,并且PI3K抑制剂LY294002显着增强了这种作用。同时,黄连显着增加了线粒体活性氧(ROS)的产生和线粒体调节蛋白向线粒体的募集。此外,自噬体形成的抑制剂没有。此外,我们的结果表明,尽管黄连上调了部分诱导LC3-I / II的AMP活化蛋白激酶(AMPK),但黄连介导的AMPK信号并未直接调节自噬细胞的死亡。此外,我们发现黄连抑制磷酸肌醇3-激酶/蛋白激酶B /雷帕霉素的哺乳动物靶标(PI3K / Akt / mTOR)的磷酸化,并且PI3K抑制剂LY294002显着增强了这种作用。同时,黄连显着增加了线粒体活性氧(ROS)的产生和线粒体调节蛋白向线粒体的募集。此外,自噬体形成的抑制剂没有。此外,我们的结果表明,尽管黄连上调了部分诱导LC3-I / II的AMP活化蛋白激酶(AMPK),但黄连介导的AMPK信号并未直接调节自噬细胞的死亡。此外,我们发现黄连抑制磷酸肌醇3-激酶/蛋白激酶B /雷帕霉素的哺乳动物靶标(PI3K / Akt / mTOR)的磷酸化,并且PI3K抑制剂LY294002显着增强了这种作用。同时,黄连显着增加了线粒体活性氧(ROS)的产生和线粒体调节蛋白向线粒体的募集。此外,黄连介导的AMPK信号并不直接调节自噬细胞的死亡。此外,我们发现黄连抑制磷酸肌醇3-激酶/蛋白激酶B /雷帕霉素的哺乳动物靶标(PI3K / Akt / mTOR)的磷酸化,并且PI3K抑制剂LY294002显着增强了这种作用。同时,黄连显着增加了线粒体活性氧(ROS)的产生和线粒体调节蛋白向线粒体的募集。此外,黄连介导的AMPK信号并不直接调节自噬细胞的死亡。此外,我们发现黄连抑制磷酸肌醇3-激酶/蛋白激酶B /雷帕霉素的哺乳动物靶标(PI3K / Akt / mTOR)的磷酸化,并且PI3K抑制剂LY294002显着增强了这种作用。同时,黄连显着增加了线粒体活性氧(ROS)的产生和线粒体调节蛋白向线粒体的募集。此外,黄连显着增加了线粒体活性氧(ROS)的产生和线粒体调节蛋白向线粒体的募集。此外,黄连显着增加了线粒体活性氧(ROS)的产生和线粒体调节蛋白向线粒体的募集。此外,N-乙酰半胱氨酸(一种潜在的ROS清除剂)通过黄连碱显着抑制了线粒体调控蛋白和LC3点的表达。总体而言,我们的结果表明,黄连介导的自噬细胞死亡受PI3K / Akt / mTOR信号传导以及与线粒体功能障碍相关的线粒体ROS产生的调节。综上,这些发现表明,黄连素通过诱导HCC Hep3B细胞自噬发挥其抗癌作用。









































 京公网安备 11010802027423号
京公网安备 11010802027423号