Acta Pharmaceutica Sinica B ( IF 14.7 ) Pub Date : 2020-11-20 , DOI: 10.1016/j.apsb.2020.11.009 Liang Jiang , Yuting Wang , Qian Li , Zhengchao Tu , Sihua Zhu , Sanfang Tu , Zhang Zhang , Ke Ding , Xiaoyun Lu
|
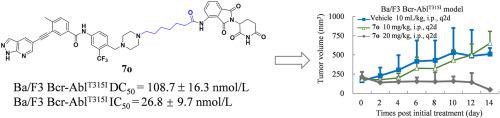
Bcr-Abl threonine 315 to isoleucine 315 (T315I) gatekeeper mutation induced drug resistance remains an unmet clinical challenge for the treatment of chronic myeloid leukemia (CML). Chemical degradation of Bcr-AblT315I protein has become a potential strategy to overcome drug resistance. Herein, we first described the design, synthesis, and evaluation of a new class of selective Bcr-AblT315I proteolysis-targeting chimeric (PROTAC) degraders based on GZD824 (reported as Bcr-AblT315I inhibitor by our group). One of the degrader 7o with 6-member carbon chain linkage with pomalidomide exhibits the most potent degradation efficacy with DR of 69.89% and 94.23% at 100 and 300 nmol/L, respectively, and has an IC50 value of 26.8 ± 9.7 nmol/L against Ba/F3T315I cells. Further, 7o also displays substantial tumor regression against Ba/F3-Bcr-AblT315I xenograft model in vivo.
中文翻译:

Bcr-Abl PROTAC克服T315I突变的设计,合成和生物学评估
从Bcr-Abl苏氨酸315到异亮氨酸315(T315I)关守突变诱导的耐药性仍然是治疗慢性粒细胞白血病(CML)的临床挑战。Bcr-Abl T315I蛋白的化学降解已成为克服耐药性的潜在策略。在本文中,我们首先描述了基于GZD824的新型一类选择性Bcr-Abl T315I蛋白水解靶向嵌合(PROTAC)降解剂的设计,合成和评估(我们小组将其称为Bcr-Abl T315I抑制剂)。具有与pomalidomide的6员碳链键合的7o降解物之一显示出最有效的降解功效,在100和300 nmol / L时的DR分别为69.89%和94.23%,并且具有IC 50相对于Ba / F3 T315I细胞的26.8±9.7 nmol / L值。此外,7o还显示出针对体内Ba / F3-Bcr-Abl T315I异种移植模型的实质性肿瘤消退。











































 京公网安备 11010802027423号
京公网安备 11010802027423号