当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photocatalytic hydrogen evolution using a Ru(II)-bound heteroaromatic ligand as a reactive site
Dalton Transactions ( IF 3.5 ) Pub Date : 2020-11-10 , DOI: 10.1039/d0dt03546g Takuya Sawaki 1, 2, 3, 4, 5 , Tomoya Ishizuka 1, 2, 3, 4, 5 , Nanase Namura 1, 2, 3, 4, 5 , Dachao Hong 1, 2, 3, 4, 5 , Mayuko Miyanishi 5, 6, 7, 8 , Yoshihito Shiota 5, 6, 7, 8 , Hiroaki Kotani 1, 2, 3, 4, 5 , Kazunari Yoshizawa 5, 6, 7, 8 , Jieun Jung 9, 10, 11, 12 , Shunichi Fukuzumi 9, 10, 11, 12, 13 , Takahiko Kojima 1, 2, 3, 4, 5
Dalton Transactions ( IF 3.5 ) Pub Date : 2020-11-10 , DOI: 10.1039/d0dt03546g Takuya Sawaki 1, 2, 3, 4, 5 , Tomoya Ishizuka 1, 2, 3, 4, 5 , Nanase Namura 1, 2, 3, 4, 5 , Dachao Hong 1, 2, 3, 4, 5 , Mayuko Miyanishi 5, 6, 7, 8 , Yoshihito Shiota 5, 6, 7, 8 , Hiroaki Kotani 1, 2, 3, 4, 5 , Kazunari Yoshizawa 5, 6, 7, 8 , Jieun Jung 9, 10, 11, 12 , Shunichi Fukuzumi 9, 10, 11, 12, 13 , Takahiko Kojima 1, 2, 3, 4, 5
Affiliation
|
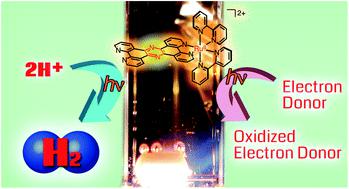
A RuII complex, [RuII(tpphz)(bpy)2]2+ (1) (tpphz = tetrapyridophenazine, bpy = 2,2′-bipyridine), whose tpphz ligand has a pyrazine moiety, is converted efficiently to [RuII(tpphz-HH)(bpy)2]2+ (2) having a dihydropyrazine moiety upon photoirradiation of a water–methanol mixed solvent solution of 1 in the presence of an electron donor. In this reaction, the triplet metal-to-ligand charge-transfer excited state (3MLCT*) of 1 is firstly formed upon photoirradiation and the 3MLCT* state is reductively quenched with an electron donor to afford [RuII(tpphz˙−)(bpy)2]+, which is converted to 2 without the observation of detectable reduced intermediates by nano-second laser flash photolysis. The inverse kinetic isotope effect (KIE) was observed to be 0.63 in the N–H bond formation of 2 at the dihydropyrazine moiety. White-light (380–670 nm) irradiation of a solution of 1 in a protic solvent, in the presence of an electron donor under an inert atmosphere, led to photocatalytic H2 evolution and the hydrogenation of organic substrates. In the reactions, complex 2 is required to be excited to form its 3MLCT* state to react with a proton and aldehydes. In photocatalytic H2 evolution, the H–H bond formation between photoexcited 2 and a proton is involved in the rate-determining step with normal KIE being 5.2 on H2 evolving rates. Density functional theory (DFT) and time-dependent DFT (TD-DFT) calculations on the reaction mechanism of H2 evolution from the ground and photo-excited states of 2 were performed to have a better understanding of the photocatalytic processes.
中文翻译:

使用Ru(II)结合的杂芳族配体作为反应位点的光催化氢释放
甲钌II络合物,的[Ru II(tpphz)(联吡啶)2 ] 2+(1)(tpphz = tetrapyridophenazine,联吡啶= 2,2'-联吡啶),其tpphz配体具有吡嗪结构部分,被有效地转化成的[Ru II(tpphz-HH)(bpy)2 ] 2+(2)在电子供体存在下,水-甲醇混合溶剂为1时,具有二氢吡嗪部分,具有二氢吡嗪部分。在该反应中,三线态的金属-配体电荷转移激发态(3的MLCT *)1在光照射和首先形成3MLCT *状态用电子给体还原性淬灭,得到[Ru II(tpphz --)(bpy)2 ] +,在没有观察到纳秒级激光闪光光解可检测到的还原中间体的情况下,将其转换为2。在二氢吡嗪部分的2的N–H键形成中,动力学逆同位素效应(KIE)为0.63 。在惰性气氛下,在电子供体存在的情况下,在质子传递溶剂中的1溶液的白光(380-670 nm)辐照,导致光催化H 2逸出和有机底物的氢化。在反应中,复杂2需要被激发以形成其3 MLCT *状态才能与质子和醛反应。在光催化H 2的演化中,光激发2与质子之间的H–H键形成参与速率确定步骤,正常KIE在H 2演化速率上为5.2 。进行了密度泛函理论(DFT)和时变DFT(TD-DFT)计算,计算了H 2从基态和2的光激发态逸出的反应机理,以更好地理解光催化过程。
更新日期:2020-11-19
中文翻译:

使用Ru(II)结合的杂芳族配体作为反应位点的光催化氢释放
甲钌II络合物,的[Ru II(tpphz)(联吡啶)2 ] 2+(1)(tpphz = tetrapyridophenazine,联吡啶= 2,2'-联吡啶),其tpphz配体具有吡嗪结构部分,被有效地转化成的[Ru II(tpphz-HH)(bpy)2 ] 2+(2)在电子供体存在下,水-甲醇混合溶剂为1时,具有二氢吡嗪部分,具有二氢吡嗪部分。在该反应中,三线态的金属-配体电荷转移激发态(3的MLCT *)1在光照射和首先形成3MLCT *状态用电子给体还原性淬灭,得到[Ru II(tpphz --)(bpy)2 ] +,在没有观察到纳秒级激光闪光光解可检测到的还原中间体的情况下,将其转换为2。在二氢吡嗪部分的2的N–H键形成中,动力学逆同位素效应(KIE)为0.63 。在惰性气氛下,在电子供体存在的情况下,在质子传递溶剂中的1溶液的白光(380-670 nm)辐照,导致光催化H 2逸出和有机底物的氢化。在反应中,复杂2需要被激发以形成其3 MLCT *状态才能与质子和醛反应。在光催化H 2的演化中,光激发2与质子之间的H–H键形成参与速率确定步骤,正常KIE在H 2演化速率上为5.2 。进行了密度泛函理论(DFT)和时变DFT(TD-DFT)计算,计算了H 2从基态和2的光激发态逸出的反应机理,以更好地理解光催化过程。











































 京公网安备 11010802027423号
京公网安备 11010802027423号