Letters in Drug Design & Discovery ( IF 1.2 ) Pub Date : 2020-11-30 , DOI: 10.2174/1570180817999200712190831 Leyla Yurttaş 1 , Asaf Evrim Evren 1 , Aslıhan Kubilay 2 , Halide Edip Temel 3 , Gülşen Akalın Çiftçi 3
|
Background: Cancer is the name given to various diseases that are mainly uncontrolled, related to cell growth and can affect various organs. Among them, lung cancer is the one, which, in its earliest stages, is difficult to diagnose, and it is asymptomatic until the disease progresses. Triazole ring is an important heterocyclic ring known with various pharmacological activities.
Objective: It is aimed to synthesize and characterize novel 1,2,4-triazole derivatives and screen them for in vitro antiproliferative activity and binding analysis through docking studies.
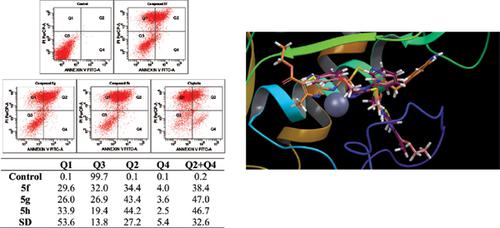
Method: In this study, we have synthesized new 2-[[5-[(4-aminophenoxy)methyl]-4-phenyl-4H- 1,2,4-triazol-3-yl]thio]-N-(substituted aryl)acetamide (5a-h) derivatives and investigated their anticancer activities against human lung cancer (A549) and mouse embryo fibroblast cell lines (NIH/3T3) by MTT, flow cytometric, caspase-3 and matrix metalloproteinase-9 (MMP-9) inhibition assays.
Results: Compounds 5f, 5g and 5h showed the highest cytotoxicity and caused significant apoptosis. These compounds inhibited MMP-9, slightly whereas they did not effect caspase-3.
Conclusion: 5f namely, N-(5-acetyl-4-methylthiazol-2-yl)-2-((5-((4-aminophenoxy)methyl)-4- phenyl-4H-1,2,4-triazol-3-yl)thio)acetamide exhibited as the most active compound with selective cytotoxicity and the highest MMP-9 inhibition. Besides, molecular modelling assessment was signified that antiproliferative activity of the compounds 5f, 5g and 5h was through a slight MMP-9 inhibition pathway.
中文翻译:

3,4,5-三取代-1,2,4-三唑衍生物作为抗增殖剂:合成,体外评估和分子模型
背景:癌症是各种疾病的名称,这些疾病主要是不受控制的,与细胞生长有关,会影响各个器官。其中,肺癌是最早的阶段,难以诊断,在疾病进展之前是无症状的。三唑环是一种重要的杂环,具有各种药理活性。
目的:旨在合成和表征新型1,2,4-三唑衍生物,并通过对接研究筛选它们的体外抗增殖活性和结合分析。
方法:在这项研究中,我们合成了新的2-[[[5-[(4-氨基苯氧基)甲基] -4-苯基-4H-1,2,4-三唑-3-基]硫代] -N-(取代芳基)乙酰胺(5a-h)衍生物,并通过MTT,流式细胞术,caspase-3和基质金属蛋白酶9(MMP-9)研究了其对人肺癌(A549)和小鼠胚胎成纤维细胞系(NIH / 3T3)的抗癌活性)抑制分析。
结果:化合物5f,5g和5h表现出最高的细胞毒性并引起明显的细胞凋亡。这些化合物略微抑制MMP-9,而它们不影响caspase-3。
结论:5f,即N-(5-乙酰基-4-甲基噻唑-2-基)-2-(((5-((4-氨基苯氧基)甲基)-4-苯基-4H-1,2,4-三唑- 3-基)硫基)乙酰胺表现出最具活性,具有选择性的细胞毒性和最高的MMP-9抑制作用。此外,分子模型评估表明,化合物5f,5g和5h的抗增殖活性是通过轻微的MMP-9抑制途径进行的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号