Letters in Drug Design & Discovery ( IF 1.2 ) Pub Date : 2020-11-30 , DOI: 10.2174/1570180817999200719152959 Sonia Verma 1 , Akashdeep Singh Pathania 1 , Somesh Baranwal 2 , Pradeep Kumar 1
|
Background: Cancer is a leading cause of deaths worldwide, accounting for 9.6 million deaths in 2018. According to the WHO, the most common causes of cancer deaths are lung, colorectal, stomach liver and breast cancer.
Introduction: PARP-1 has a crucial role in cell proliferation, survival and death due to its role in the regulation of multiple biological processes. Quinazolinone and its derivatives represent a large class of biologically active compounds that exhibit a broad spectrum of biological activities such as anti-HIV, anticancer, antifungal, antibacterial, anticonvulsant, anti-inflammatory, antidepressant, antimalarial, antioxidant and antileishmanial activities.
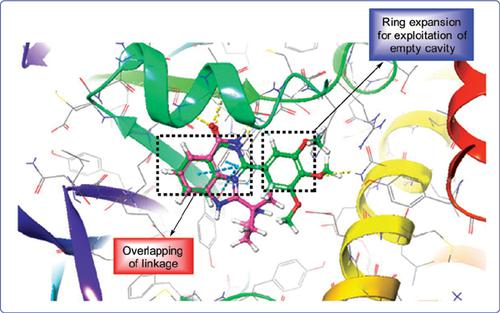
Methods: In this study, we have synthesized quinazolinone derivatives by reaction of 2- aminobenzamide and substituted benzaldehydes. The synthesized compounds were also screened in silico for their PARP-1 binding affinities by molecular docking studies using Schrodinger 2016 software. In silico ADME studies were also performed for the synthesized compounds by using QikProp tool of Schrodinger software.
Results: Results of in silico studies indicated that quinazolinone derivatives exhibited a good affinity towards the active site of PARP-1. Out of all synthesized compounds, SVA-11 exhibited a maximum dock score (-10.421). Results of ADME studies indicated the suitability of synthesized compounds as drug candidates.
Conclusion: The synthesized compounds showed better docking scores than reference drug valiparib. Furthermore, they exhibited favorable ADME profile. Therefore, they may serve as lead compounds in the discovery of PARP-1 inhibitors.
中文翻译:

喹唑啉酮衍生物作为PARP-1抑制剂的合成和计算机模拟研究
背景:癌症是全球主要的死亡原因,在2018年占960万人的死亡。根据WHO的说法,最常见的癌症死亡原因是肺癌,大肠癌,胃肝癌和乳腺癌。
简介:PARP-1在调节多种生物过程中起着重要的作用,在细胞增殖,存活和死亡中起着至关重要的作用。喹唑啉酮及其衍生物代表一类具有广泛生物活性的生物活性化合物,例如抗HIV,抗癌,抗真菌,抗菌,抗惊厥,抗炎,抗抑郁,抗疟疾,抗氧化剂和抗衰老活性。
方法:在这项研究中,我们通过2-氨基苯甲酰胺和取代的苯甲醛的反应合成了喹唑啉酮衍生物。还使用Schrodinger 2016软件通过分子对接研究在计算机上筛选了合成的化合物的PARP-1结合亲和力。使用Schrodinger软件的QikProp工具对合成的化合物进行了计算机模拟ADME研究。
结果:计算机研究的结果表明,喹唑啉酮衍生物对PARP-1的活性位点具有良好的亲和力。在所有合成的化合物中,SVA-11的最大对接得分(-10.421)。ADME研究的结果表明合成的化合物适合作为候选药物。
结论:合成的化合物显示出比参比药物伐利帕利更好的对接分数。此外,它们表现出良好的ADME特性。因此,它们可以作为PARP-1抑制剂发现中的先导化合物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号