Letters in Drug Design & Discovery ( IF 1.2 ) Pub Date : 2020-11-30 , DOI: 10.2174/1570180817999200706005247 Akhil Bansal 1 , Alka Bali 1 , Ajitesh Balaini 1
|
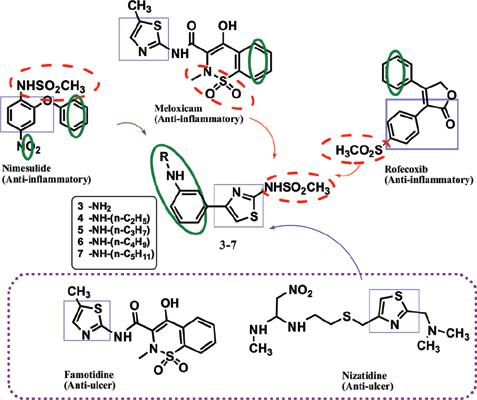
Background: NSAIDs are used as first-line drugs for the treatment of various inflammatory disorders. Chronic use of NSAIDs is known to be associated with gastrointestinal and renal toxicity. Local generation of reactive oxygen species finally resulting in cellular apoptosis is one of the accepted mechanisms for NSAID-induced toxicity.
Objective: The objective of the present study was to design and synthesize a series of 2-methane sulfonamido substituted arylthiazole derivatives by including structural features of combined antiulcer and anti-inflammatory activity utilizing as the structural core, thiazole nucleus with potential for antioxidant effect.
Methods: Compounds were designed based on three dimensional and field similarity studies. The synthesized compounds were evaluated for their anti-inflammatory activity in carrageenan-induced rat paw edema model. Rofecoxib and indomethacin were taken as standard drugs for comparison. The in vitro antioxidant activity was assessed in potassium ferricyanide reducing power (PFRAP) assay employing ascorbic acid as the standard drug.
Results: The compounds 6 and 7 showed good anti-inflammatory activity comparable to the standard group and were also non ulcerogenic at the test doses. Compounds 1-7 displayed varying degrees of reducing power in the PFRAP) assay and the methanesulphonamido derivatives 4-7 showed the highest antioxidant activity (EC50 values 3.7-5.1 μmol/ml vs ascorbic acid 7.4 μmol/ml). Theoretical ADME profiling of the compounds based on selected physicochemical properties showed excellent compliance with Lipinski’s rule.
Conclusion: A series of compounds have been designed and synthesized having dual antioxidant and anti-inflammatory activity with activities comparable to standard drugs.
中文翻译:

具有抗氧化潜力的保芳抗炎药取代的芳基噻唑的合成与评价
背景:非甾体抗炎药被用作治疗各种炎性疾病的一线药物。长期使用非甾体抗炎药与胃肠道和肾脏毒性有关。最终导致细胞凋亡的活性氧的局部生成是NSAID诱导毒性的公认机制之一。
目的:本研究的目的是通过结合噻唑核具有抗溃疡和消炎活性的结构特征,设计和合成一系列2-甲烷磺酰胺基取代的芳基噻唑衍生物,噻唑核具有抗氧化作用的潜力。
方法:基于三维和领域相似性研究设计化合物。在角叉菜胶诱导的大鼠爪水肿模型中评估了合成的化合物的抗炎活性。罗非考昔和消炎痛被作为标准药物进行比较。使用抗坏血酸作为标准药物,在铁氰化钾还原能力(PFRAP)分析中评估了体外抗氧化活性。
结果:化合物6和7表现出与标准组相当的良好抗炎活性,并且在测试剂量下也不致溃疡。化合物1-7在PFRAP)分析中显示出不同程度的还原能力,甲烷磺酰胺基衍生物4-7显示出最高的抗氧化活性(EC50值为3.7-5.1μmol/ ml,抗坏血酸为7.4μmol/ ml)。根据选定的理化性质对化合物进行理论上的ADME分析,显示出极好的Lipinski规则。
结论:已设计并合成了具有双重抗氧化和抗炎活性且具有与标准药物相当的活性的一系列化合物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号