当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Hydration-Induced Disorder Lowers the Energy Barriers for Methyl Rotation in Drug Molecules
The Journal of Physical Chemistry Letters ( IF 5.7 ) Pub Date : 2020-11-19 , DOI: 10.1021/acs.jpclett.0c02642 Eugene Mamontov 1 , Yongqiang Cheng 1 , Luke L. Daemen 1 , Alexander I. Kolesnikov 1 , Anibal J. Ramirez-Cuesta 1 , Matthew R. Ryder 1 , Matthew B. Stone 1
The Journal of Physical Chemistry Letters ( IF 5.7 ) Pub Date : 2020-11-19 , DOI: 10.1021/acs.jpclett.0c02642 Eugene Mamontov 1 , Yongqiang Cheng 1 , Luke L. Daemen 1 , Alexander I. Kolesnikov 1 , Anibal J. Ramirez-Cuesta 1 , Matthew R. Ryder 1 , Matthew B. Stone 1
Affiliation
|
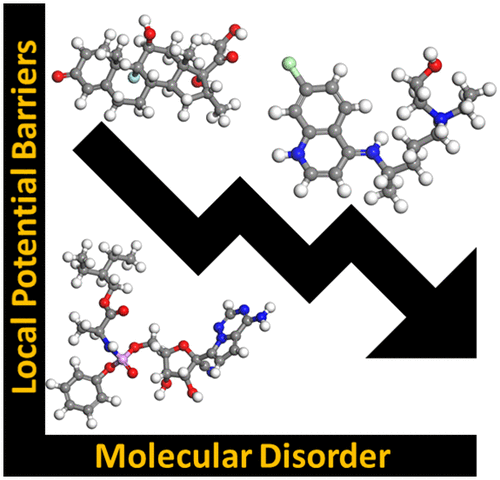
The thermally activated dynamics of methyl groups are important for biochemical activity as they allow for a more efficient sampling of the energy landscape. Here, we compare methyl rotations in the dry and variously hydrated states of three primary drugs under consideration to treat the recent coronavirus disease (COVID-19), namely, hydroxychloroquine and its sulfate, dexamethasone and its sodium diphosphate, and remdesivir. We find that the main driving force behind the considerable reduction in the activation energy for methyl rotations in the hydrated state is the hydration-induced disorder in the methyl group local environments. Furthermore, the activation energy for methyl rotations in the hydration-induced disordered state is much lower than that in an isolated drug molecule, indicating that neither isolated molecules nor periodic crystalline structures can be used to analyze the potential landscape governing the side group dynamics in drug molecules. Instead, only the explicitly considered disordered structures can provide insight.
中文翻译:

水合诱发的疾病降低了药物分子中甲基旋转的能量屏障。
甲基的热活化动力学对于生化活性很重要,因为它们可以更有效地采样能量分布。在这里,我们比较了正在考虑治疗最近的冠状病毒疾病(COVID-19)的三种主要药物在干燥和水合状态下的甲基旋转,它们是羟氯喹及其硫酸盐,地塞米松及其二磷酸钠和瑞姆昔韦。我们发现,在水合状态下甲基旋转的活化能显着降低的主要驱动力是在甲基局部环境中水合诱导的紊乱。此外,在水合诱导的无序状态下,甲基旋转的活化能比在分离的药物分子中低得多,表明分离的分子和周期性的晶体结构都不能用于分析控制药物分子中侧基动力学的潜在态势。相反,只有明确考虑的无序结构才能提供洞察力。
更新日期:2020-12-03
中文翻译:

水合诱发的疾病降低了药物分子中甲基旋转的能量屏障。
甲基的热活化动力学对于生化活性很重要,因为它们可以更有效地采样能量分布。在这里,我们比较了正在考虑治疗最近的冠状病毒疾病(COVID-19)的三种主要药物在干燥和水合状态下的甲基旋转,它们是羟氯喹及其硫酸盐,地塞米松及其二磷酸钠和瑞姆昔韦。我们发现,在水合状态下甲基旋转的活化能显着降低的主要驱动力是在甲基局部环境中水合诱导的紊乱。此外,在水合诱导的无序状态下,甲基旋转的活化能比在分离的药物分子中低得多,表明分离的分子和周期性的晶体结构都不能用于分析控制药物分子中侧基动力学的潜在态势。相反,只有明确考虑的无序结构才能提供洞察力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号