当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Theoretical Investigation of the Inhibitory Mechanism of Norepinephrine on hIAPP Amyloid Aggregation and the Destabilization of Protofibrils
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-11-18 , DOI: 10.1021/acs.jpcb.0c07830 Rituparna Roy 1 , Sandip Paul 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-11-18 , DOI: 10.1021/acs.jpcb.0c07830 Rituparna Roy 1 , Sandip Paul 1
Affiliation
|
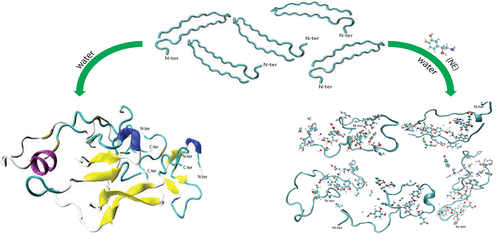
The search for an appropriate drug to completely eradicate type II diabetes (T2D), a metabolic disorder from which over 40 million people suffer worldwide, has not yet led to any satisfactory result. The misfolding of human islet amyloid polypeptide (hIAPP) into toxic oligomers is a pathogenic feature of this disease, due to which the prevention of hIAPP aggregation is considered the rational approach to combat T2D. Hence, we study the role of a catecholamine, norepinephrine, on the amyloid aggregation of hIAPP, which has previously displayed inhibitory effect on amyloid-β aggregation. Via all-atom molecular dynamics simulations, we observe that norepinephrine can not only inhibit the aggregation of hIAPP but also partially disassemble the preformed fibrils. For comparison, the influence of two other molecules (aspirin and benzimidazole, both of which have previously reported to have no inhibitory impact on hIAPP aggregation) is also analyzed. We observe that the conformational preference of hIAPP changes from a β-sheet conformation to a disordered state when norepinephrine is added to the peptides. However, no such effect is observed in the presence of aspirin or benzimidazole. In-depth investigation reveals that the β-sheets formed between Leu12–His18 and Leu27–Gly33 enhance the peptide–peptide interactions that are broken by norepinephrine, which itself interacts with the peptides via hydrogen bonding, hydrophobic, and aromatic stacking interactions, preferentially with the C-terminal residues of hIAPP. The molecular mechanism action of norepinephrine on hIAPP aggregation can provide useful insight for the drug design against T2D.
中文翻译:

去甲肾上腺素抑制hIAPP淀粉样蛋白聚集和原纤维去稳定作用机理的理论研究
寻找一种可以完全根除II型糖尿病(T2D)的合适药物的方法尚未获得令人满意的结果,该疾病是全球超过4000万人罹患的代谢性疾病。人胰岛淀粉样多肽(hIAPP)错折叠为有毒的寡聚体是该疾病的致病特征,因此,预防hIAPP聚集被认为是对抗T2D的合理方法。因此,我们研究了儿茶酚胺去甲肾上腺素对hIAPP的淀粉样蛋白聚集的作用,该蛋白先前已显示出对淀粉样蛋白β聚集的抑制作用。通过全原子分子动力学模拟,我们观察到去甲肾上腺素不仅可以抑制hIAPP的聚集,而且可以部分分解预制的原纤维。为了比较,其他两种分子(阿司匹林和苯并咪唑,还分析了先前报道的这两种对hIAPP聚集均无抑制作用的物质。我们观察到,当将去甲肾上腺素添加到肽中时,hIAPP的构象偏好从β-折叠构象变为无序状态。但是,在阿司匹林或苯并咪唑的存在下没有观察到这种作用。深入研究表明,Leu12–His18和Leu27–Gly33之间形成的β-折叠增强了被去甲肾上腺素破坏的肽-肽相互作用,去甲肾上腺素本身通过氢键,疏水和芳族堆积相互作用与肽相互作用,优先与hIAPP的C端残基。去甲肾上腺素对hIAPP聚集的分子机制作用可为针对T2D的药物设计提供有用的见解。
更新日期:2020-12-03
中文翻译:

去甲肾上腺素抑制hIAPP淀粉样蛋白聚集和原纤维去稳定作用机理的理论研究
寻找一种可以完全根除II型糖尿病(T2D)的合适药物的方法尚未获得令人满意的结果,该疾病是全球超过4000万人罹患的代谢性疾病。人胰岛淀粉样多肽(hIAPP)错折叠为有毒的寡聚体是该疾病的致病特征,因此,预防hIAPP聚集被认为是对抗T2D的合理方法。因此,我们研究了儿茶酚胺去甲肾上腺素对hIAPP的淀粉样蛋白聚集的作用,该蛋白先前已显示出对淀粉样蛋白β聚集的抑制作用。通过全原子分子动力学模拟,我们观察到去甲肾上腺素不仅可以抑制hIAPP的聚集,而且可以部分分解预制的原纤维。为了比较,其他两种分子(阿司匹林和苯并咪唑,还分析了先前报道的这两种对hIAPP聚集均无抑制作用的物质。我们观察到,当将去甲肾上腺素添加到肽中时,hIAPP的构象偏好从β-折叠构象变为无序状态。但是,在阿司匹林或苯并咪唑的存在下没有观察到这种作用。深入研究表明,Leu12–His18和Leu27–Gly33之间形成的β-折叠增强了被去甲肾上腺素破坏的肽-肽相互作用,去甲肾上腺素本身通过氢键,疏水和芳族堆积相互作用与肽相互作用,优先与hIAPP的C端残基。去甲肾上腺素对hIAPP聚集的分子机制作用可为针对T2D的药物设计提供有用的见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号