当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Long-Range Charge Reorganization as an Allosteric Control Signal in Proteins
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-11-19 , DOI: 10.1021/jacs.0c10105 Koyel Banerjee-Ghosh 1 , Shirsendu Ghosh 1 , Hisham Mazal 1 , Inbal Riven 1 , Gilad Haran 1 , Ron Naaman 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-11-19 , DOI: 10.1021/jacs.0c10105 Koyel Banerjee-Ghosh 1 , Shirsendu Ghosh 1 , Hisham Mazal 1 , Inbal Riven 1 , Gilad Haran 1 , Ron Naaman 1
Affiliation
|
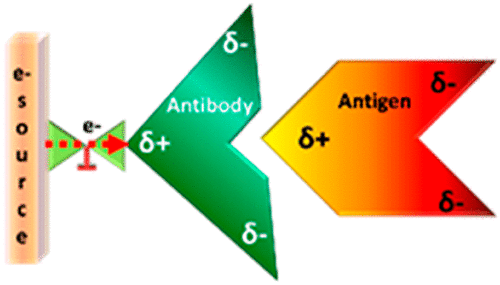
A new mechanism of allostery in proteins, based on charge rather than structure, is reported. We demonstrate that dynamic redistribution of charge within a protein can control its function and affect its interaction with a binding partner. In particular, the association of an antibody with its target protein antigen is studied. Dynamic charge shifting within the antibody during its interaction with the antigen is enabled by its binding to a metallic surface that serves as a source for electrons. The kinetics of antibody–antigen association are enhanced when charge redistribution is allowed, even though charge injection happens at a position far from the antigen binding site. This observation points to charge-reorganization allostery, which should be operative in addition or parallel to other mechanisms of allostery, and may explain some current observations on protein interactions.
中文翻译:

远程电荷重组作为蛋白质中的变构控制信号
报告了一种基于电荷而非结构的蛋白质变构新机制。我们证明蛋白质内电荷的动态重新分配可以控制其功能并影响其与结合伙伴的相互作用。特别是,研究了抗体与其靶蛋白抗原的关联。在抗体与抗原相互作用期间,抗体内的动态电荷转移是通过其与作为电子源的金属表面结合而实现的。当允许电荷重新分布时,抗体-抗原结合的动力学会增强,即使电荷注入发生在远离抗原结合位点的位置。这一观察指向电荷重组变构,它应该与其他变构机制相辅相成或与之并行,
更新日期:2020-11-19
中文翻译:

远程电荷重组作为蛋白质中的变构控制信号
报告了一种基于电荷而非结构的蛋白质变构新机制。我们证明蛋白质内电荷的动态重新分配可以控制其功能并影响其与结合伙伴的相互作用。特别是,研究了抗体与其靶蛋白抗原的关联。在抗体与抗原相互作用期间,抗体内的动态电荷转移是通过其与作为电子源的金属表面结合而实现的。当允许电荷重新分布时,抗体-抗原结合的动力学会增强,即使电荷注入发生在远离抗原结合位点的位置。这一观察指向电荷重组变构,它应该与其他变构机制相辅相成或与之并行,











































 京公网安备 11010802027423号
京公网安备 11010802027423号