当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Monoradicals and Diradicals of Dibenzofluoreno[3,2-b]fluorene Isomers: Mechanisms of Electronic Delocalization
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-11-18 , DOI: 10.1021/jacs.0c09588 Hideki Hayashi 1 , Joshua E Barker 2 , Abel Cárdenas Valdivia 3 , Ryohei Kishi 4, 5 , Samantha N MacMillan 6 , Carlos J Gómez-García 7 , Hidenori Miyauchi 8 , Yosuke Nakamura 8 , Masayoshi Nakano 4, 5, 9 , Shin-Ichiro Kato 1 , Michael M Haley 2 , Juan Casado 3
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-11-18 , DOI: 10.1021/jacs.0c09588 Hideki Hayashi 1 , Joshua E Barker 2 , Abel Cárdenas Valdivia 3 , Ryohei Kishi 4, 5 , Samantha N MacMillan 6 , Carlos J Gómez-García 7 , Hidenori Miyauchi 8 , Yosuke Nakamura 8 , Masayoshi Nakano 4, 5, 9 , Shin-Ichiro Kato 1 , Michael M Haley 2 , Juan Casado 3
Affiliation
|
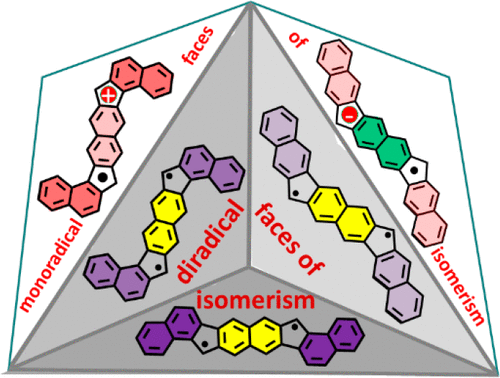
The preparation of a series of dibenzo- and tetrabenzo-fused fluoreno[3,2-b]fluorenes is disclosed, and the diradicaloid properties of these molecules are compared with those of a similar, previously reported series of anthracene-based diradicaloids. Insights on the diradical mode of delocalization tuning by constitutional isomerism of the external naphthalenes has been explored by means of the physical approach (dissection of the electronic properties in terms of electronic repulsion and transfer integral) of diradicals. This study has also been extended to the redox species of the two series of compounds and found that the radical cations have the same stabilization mode by delocalization that the neutral diradicals while the radical anions, contrarily, are stabilized by aromatization of the central core. The synthesis of the fluorenofluorene series and their characterization by electronic absorption and vibrational Raman spectroscopies, X-ray diffraction, SQUID measurements, electrochemistry, in situ UV-vis-NIR absorption spectroelectrochemistry, and theoretical calculations are presented. This work attempts to unify the properties of different series of diradicaloids in a common argument as well as the properties of the carbocations and carbanions derived from them.
中文翻译:

二苯并芴[3,2-b]芴异构体的单自由基和双自由基:电子离域机制
公开了一系列二苯并和四苯并稠合芴[3,2-b]芴的制备,并将这些分子的双自由基性质与先前报道的一系列类似的基于蒽的双自由基性质进行了比较。通过双自由基的物理方法(根据电子排斥和转移积分来剖析电子特性),探索了通过外部萘的结构异构性进行离域调节的双自由基模式的见解。这项研究还扩展到两个系列化合物的氧化还原物质,发现自由基阳离子具有与中性双自由基相同的离域稳定模式,而自由基阴离子则相反,通过中心核的芳构化来稳定。介绍了芴基芴系列的合成及其通过电子吸收和振动拉曼光谱、X 射线衍射、SQUID 测量、电化学、原位紫外-可见-近红外吸收光谱电化学和理论计算的表征。这项工作试图将不同系列双自由基的性质以及源自它们的碳正离子和碳负离子的性质统一在一个共同的论点中。
更新日期:2020-11-18
中文翻译:

二苯并芴[3,2-b]芴异构体的单自由基和双自由基:电子离域机制
公开了一系列二苯并和四苯并稠合芴[3,2-b]芴的制备,并将这些分子的双自由基性质与先前报道的一系列类似的基于蒽的双自由基性质进行了比较。通过双自由基的物理方法(根据电子排斥和转移积分来剖析电子特性),探索了通过外部萘的结构异构性进行离域调节的双自由基模式的见解。这项研究还扩展到两个系列化合物的氧化还原物质,发现自由基阳离子具有与中性双自由基相同的离域稳定模式,而自由基阴离子则相反,通过中心核的芳构化来稳定。介绍了芴基芴系列的合成及其通过电子吸收和振动拉曼光谱、X 射线衍射、SQUID 测量、电化学、原位紫外-可见-近红外吸收光谱电化学和理论计算的表征。这项工作试图将不同系列双自由基的性质以及源自它们的碳正离子和碳负离子的性质统一在一个共同的论点中。











































 京公网安备 11010802027423号
京公网安备 11010802027423号