当前位置:
X-MOL 学术
›
Cryst. Growth Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Time-Resolved Cooperative Motions in the Solid-State Dehydration of Thymine Hydrate
Crystal Growth & Design ( IF 3.8 ) Pub Date : 2020-11-19 , DOI: 10.1021/acs.cgd.0c01210 Taylor A. Watts 1 , Elizabeth K. Miehls 1 , Jennifer A. Swift 1
Crystal Growth & Design ( IF 3.8 ) Pub Date : 2020-11-19 , DOI: 10.1021/acs.cgd.0c01210 Taylor A. Watts 1 , Elizabeth K. Miehls 1 , Jennifer A. Swift 1
Affiliation
|
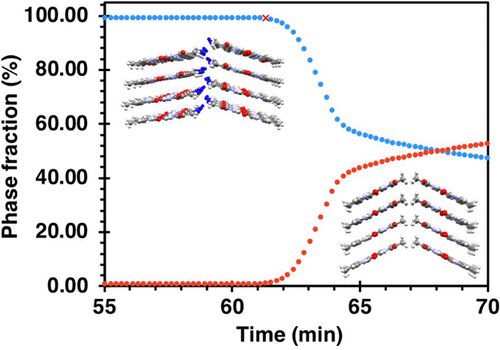
Many organic compounds can exist in hydrated and anhydrous crystalline forms, each of which exhibits its own different set of physical properties. This makes dehydration–rehydration processes an important class of solid-state reactions; yet, the molecular-level mechanisms and cooperative motions which govern such transformation in molecular solids have been notoriously difficult to establish. Here using time-resolved synchrotron X-ray powder diffraction (sPXRD) and other methods, we identify a number of early subtle changes in thymine hydrate (TH) which set its trajectory for the formation of anhydrous products. An early cooperative “morning stretch” motion, characterized by a coordinated increase in the interlayer separation and angular rotation, was observed prior to the appearance of the first major anhydrous phase, Td1. Kinetic analyses indicated the overall solid-state reaction proceeds via a one-dimensional diffusion mechanism with an Ea = 115–122 kJ/mol. At temperatures ≥45 °C, solid state dehydration yielded mixtures of Td1 and a second major anhydrous phase, Td2. Multiphase refinement of sPXRD data proved that Td2 is formed via two distinct routes—either directly due to water loss from TH or via the polymorphic transformation of Td1. Heating above ∼180 °C yielded Td2 as the major product. A limited number of weak diffraction peaks evidence the presence of a third transient form, Td*, which also converts to Td2. The time-resolved methods used here illustrate that solid-state dehydration even in a seemingly simple molecular hydrate system involves a significantly more complex set of coordinated molecular motions and solid–solid transformations than originally thought. The ability to glean a more complete picture of the cooperative motions that occur during water loss from soft crystalline hydrates is an important step in the development of a deeper understanding of this important class of materials.
中文翻译:

胸腺嘧啶水合物固态脱水中的时间分辨合作运动
许多有机化合物可以以水合和无水结晶形式存在,每种形式都表现出不同的物理性质。这使得脱水-再水化过程成为一类重要的固态反应。然而,众所周知,难以控制支配分子固体中这种转变的分子级机理和协同运动。在这里,使用时间分辨的同步加速器X射线粉末衍射(sPXRD)和其他方法,我们确定了胸腺嘧啶水合物(TH)的许多早期细微变化,从而改变了其形成无水产物的轨迹。在出现第一个主要无水相Td 1之前,观察到了早期的协作“早晨拉伸”运动,其特征是层间分离和角旋转的协调增加。。动力学分析表明,整个固态反应通过一维扩散机制进行,E a = 115–122 kJ / mol。在≥45°C的温度下,固态脱水会生成Td 1和第二主要无水相Td 2的混合物。sPXRD数据的多阶段精炼证明,Td 2是通过两条截然不同的途径形成的-要么直接由于TH的水分流失,要么通过Td 1的多态转化。加热到约180°C以上,生成Td 2作为主要产物。的弱衍射峰证据数量有限的第三瞬态形式的存在和Td *,这也转换到Td的2。这里使用的时间分辨方法说明,即使在看似简单的分子水合物系统中,固态脱水也比原先想象的要复杂得多,这包括一组协调的分子运动和固-固转化。收集对软结晶水合物失水过程中发生的协同运动的更完整了解的能力,是对这种重要材料类型有更深入了解的重要一步。
更新日期:2020-12-02
中文翻译:

胸腺嘧啶水合物固态脱水中的时间分辨合作运动
许多有机化合物可以以水合和无水结晶形式存在,每种形式都表现出不同的物理性质。这使得脱水-再水化过程成为一类重要的固态反应。然而,众所周知,难以控制支配分子固体中这种转变的分子级机理和协同运动。在这里,使用时间分辨的同步加速器X射线粉末衍射(sPXRD)和其他方法,我们确定了胸腺嘧啶水合物(TH)的许多早期细微变化,从而改变了其形成无水产物的轨迹。在出现第一个主要无水相Td 1之前,观察到了早期的协作“早晨拉伸”运动,其特征是层间分离和角旋转的协调增加。。动力学分析表明,整个固态反应通过一维扩散机制进行,E a = 115–122 kJ / mol。在≥45°C的温度下,固态脱水会生成Td 1和第二主要无水相Td 2的混合物。sPXRD数据的多阶段精炼证明,Td 2是通过两条截然不同的途径形成的-要么直接由于TH的水分流失,要么通过Td 1的多态转化。加热到约180°C以上,生成Td 2作为主要产物。的弱衍射峰证据数量有限的第三瞬态形式的存在和Td *,这也转换到Td的2。这里使用的时间分辨方法说明,即使在看似简单的分子水合物系统中,固态脱水也比原先想象的要复杂得多,这包括一组协调的分子运动和固-固转化。收集对软结晶水合物失水过程中发生的协同运动的更完整了解的能力,是对这种重要材料类型有更深入了解的重要一步。



























 京公网安备 11010802027423号
京公网安备 11010802027423号