当前位置:
X-MOL 学术
›
Eur. J. Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Preparation and Optical and Electrochemical Properties of Boron (III) Subphthalocyanines with One to Three Trithiole Rings
European Journal of Inorganic Chemistry ( IF 2.2 ) Pub Date : 2020-11-18 , DOI: 10.1002/ejic.202000862 Takeshi Kimura 1 , Mizue Baba 1
European Journal of Inorganic Chemistry ( IF 2.2 ) Pub Date : 2020-11-18 , DOI: 10.1002/ejic.202000862 Takeshi Kimura 1 , Mizue Baba 1
Affiliation
|
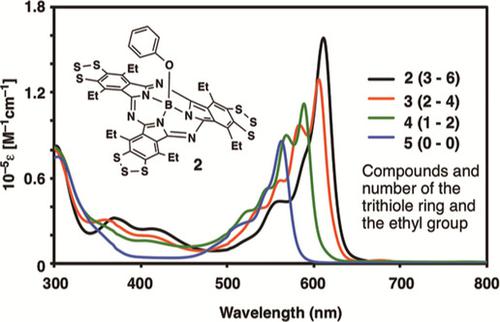
The reaction of 5,6‐dicyano‐4,7‐diethylbenzo[1,2,3]trithiole (1) with trichloroborane in p‐xylene and subsequent substitution of the chloro group with phenol produced boron (III) subphthalocyanine 2 with three trithiole rings. Upon treatment of 1 with trichloroborane in p‐xylene in the presence of unsubstituted phthalonitrile or tetrafluorophthalonitrile, unsymmetrical subphthalocyanines 3, 4, 6, and 7 with one or two trithiole rings were obtained after the reaction with phenol. The Q‐band absorption of 2 appeared at λmax=611 nm in the UV‐vis spectrum and the emission was observed at λe=630 nm. Decreasing the number of fused trithiole rings and ethyl groups resulted in higher energy shifts of the Q‐band absorption in the UV‐vis spectra and downfield chemical shifts of the 11B NMR signals. Compounds 2, 3, and 4 were treated by pentachloro antimonate in dichloromethane, the solution of which showed strong ESR signals. The structures of simplified model compounds were optimized using the DFT method with the Gaussian 09 program at the B3LYP/6‐31G (d, p) level.
中文翻译:

具有1-3个三硫醇环的硼(III)亚酞菁的制备及其光学和电化学性能
5,6-二氰基-4,7-二乙基苯并反应[1,2,3] trithiole(1)与三氯硼烷p二甲苯和氯基团的后续取代与苯酚产生硼(III)的亚酞菁2具有三个trithiole戒指。处理后的1与三氯硼烷在p二甲苯在未取代的邻苯二甲腈或tetrafluorophthalonitrile,不对称的亚酞菁的存在3,4,6,和7与一个或两个环trithiole与苯酚在反应后得到的。Q带吸收2出现在λmax=在UV-Vis光谱611纳米,发射在λ观察Ê = 630纳米。减少稠合的三硫醇环和乙基的数量会导致UV可见光谱中Q波段吸收的能量转移更高,以及11 B NMR信号的低场化学位移。化合物2,3和4是由五氯锑酸盐在二氯甲烷处理,其中所述溶液表现出较强的ESR信号。使用DFT方法和高斯09程序在B3LYP / 6-31G(d,p)水平上对简化模型化合物的结构进行了优化。
更新日期:2020-11-18
中文翻译:

具有1-3个三硫醇环的硼(III)亚酞菁的制备及其光学和电化学性能
5,6-二氰基-4,7-二乙基苯并反应[1,2,3] trithiole(1)与三氯硼烷p二甲苯和氯基团的后续取代与苯酚产生硼(III)的亚酞菁2具有三个trithiole戒指。处理后的1与三氯硼烷在p二甲苯在未取代的邻苯二甲腈或tetrafluorophthalonitrile,不对称的亚酞菁的存在3,4,6,和7与一个或两个环trithiole与苯酚在反应后得到的。Q带吸收2出现在λmax=在UV-Vis光谱611纳米,发射在λ观察Ê = 630纳米。减少稠合的三硫醇环和乙基的数量会导致UV可见光谱中Q波段吸收的能量转移更高,以及11 B NMR信号的低场化学位移。化合物2,3和4是由五氯锑酸盐在二氯甲烷处理,其中所述溶液表现出较强的ESR信号。使用DFT方法和高斯09程序在B3LYP / 6-31G(d,p)水平上对简化模型化合物的结构进行了优化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号