Molecular Cell ( IF 14.5 ) Pub Date : 2020-11-19 , DOI: 10.1016/j.molcel.2020.10.032 Hagai Marmor-Kollet 1 , Aviad Siany 1 , Nancy Kedersha 2 , Naama Knafo 3 , Natalia Rivkin 1 , Yehuda M Danino 1 , Thomas G Moens 4 , Tsviya Olender 1 , Daoud Sheban 5 , Nir Cohen 1 , Tali Dadosh 6 , Yoseph Addadi 7 , Revital Ravid 1 , Chen Eitan 1 , Beata Toth Cohen 1 , Sarah Hofmann 2 , Claire L Riggs 2 , Vivek M Advani 2 , Adrian Higginbottom 8 , Johnathan Cooper-Knock 8 , Jacob H Hanna 1 , Yifat Merbl 9 , Ludo Van Den Bosch 4 , Paul Anderson 2 , Pavel Ivanov 2 , Tamar Geiger 10 , Eran Hornstein 1
|
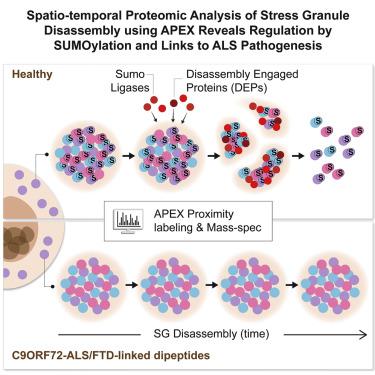
Stress granules (SGs) are cytoplasmic assemblies of proteins and non-translating mRNAs. Whereas much has been learned about SG formation, a major gap remains in understanding the compositional changes SGs undergo during normal disassembly and under disease conditions. Here, we address this gap by proteomic dissection of the SG temporal disassembly sequence using multi-bait APEX proximity proteomics. We discover 109 novel SG proteins and characterize distinct SG substructures. We reveal dozens of disassembly-engaged proteins (DEPs), some of which play functional roles in SG disassembly, including small ubiquitin-like modifier (SUMO) conjugating enzymes. We further demonstrate that SUMOylation regulates SG disassembly and SG formation. Parallel proteomics with amyotrophic lateral sclerosis (ALS)-associated C9ORF72 dipeptides uncovered attenuated DEP recruitment during SG disassembly and impaired SUMOylation. Accordingly, SUMO activity ameliorated C9ORF72-ALS-related neurodegeneration in Drosophila. By dissecting the SG spatiotemporal proteomic landscape, we provide an in-depth resource for future work on SG function and reveal basic and disease-relevant mechanisms of SG disassembly.
中文翻译:

使用 APEX 对应力颗粒分解的时空蛋白质组学分析揭示了 SUMO 化的调节以及与 ALS 发病机制的联系
应激颗粒 (SG) 是蛋白质和非翻译 mRNA 的细胞质组装体。尽管人们对 SG 的形成了解很多,但在理解 SG 在正常分解过程中和疾病条件下所经历的成分变化方面仍然存在重大差距。在这里,我们通过使用多诱饵 APEX 邻近蛋白质组学对 SG 时间分解序列进行蛋白质组学解剖来解决这一差距。我们发现了 109 种新型 SG 蛋白并表征了不同的 SG 子结构。我们揭示了数十种拆卸参与蛋白 (DEP),其中一些在 SG 拆卸中发挥功能作用,包括小型泛素样修饰剂 (SUMO) 缀合酶。我们进一步证明 SUMOylation 调节 SG 分解和 SG 形成。与肌萎缩性脊髓侧索硬化症 (ALS) 相关的 C9ORF72 二肽的平行蛋白质组学发现,SG 解体过程中 DEP 募集减弱,SUMO 化受损。因此,SUMO 活性改善了果蝇中 C9ORF72-ALS 相关的神经变性。通过剖析 SG 时空蛋白质组景观,我们为未来 SG 功能的工作提供了深入的资源,并揭示了 SG 分解的基本和疾病相关机制。










































 京公网安备 11010802027423号
京公网安备 11010802027423号