Molecular Cell ( IF 14.5 ) Pub Date : 2020-11-19 , DOI: 10.1016/j.molcel.2020.10.035 Brady A Travis 1 , Kathryn M Ramsey 2 , Samantha M Prezioso 3 , Thomas Tallo 3 , Jamie M Wandzilak 4 , Allen Hsu 5 , Mario Borgnia 5 , Alberto Bartesaghi 6 , Simon L Dove 3 , Richard G Brennan 1 , Maria A Schumacher 1
|
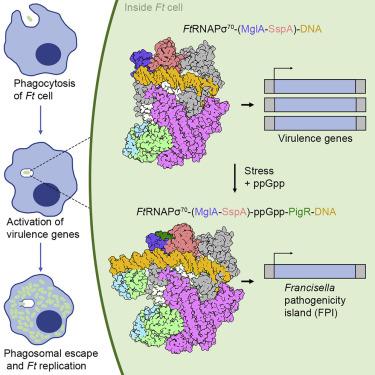
The bacterium Francisella tularensis (Ft) is one of the most infectious agents known. Ft virulence is controlled by a unique combination of transcription regulators: the MglA-SspA heterodimer, PigR, and the stress signal, ppGpp. MglA-SspA assembles with the σ70-associated RNAP holoenzyme (RNAPσ70), forming a virulence-specialized polymerase. These factors activate Francisella pathogenicity island (FPI) gene expression, which is required for virulence, but the mechanism is unknown. Here we report FtRNAPσ70-promoter-DNA, FtRNAPσ70-(MglA-SspA)-promoter DNA, and FtRNAPσ70-(MglA-SspA)-ppGpp-PigR-promoter DNA cryo-EM structures. Structural and genetic analyses show MglA-SspA facilitates σ70 binding to DNA to regulate virulence and virulence-enhancing genes. Our Escherichia coli RNAPσ70-homodimeric EcSspA structure suggests this is a general SspA-transcription regulation mechanism. Strikingly, our FtRNAPσ70-(MglA-SspA)-ppGpp-PigR-DNA structure reveals ppGpp binding to MglA-SspA tethers PigR to promoters. PigR in turn recruits FtRNAP αCTDs to DNA UP elements. Thus, these studies unveil a unique mechanism for Ft pathogenesis involving a virulence-specialized RNAP that employs two (MglA-SspA)-based strategies to activate virulence genes.
中文翻译:

土拉弗朗西斯菌毒力激活的结构基础
土拉弗朗西斯菌 ( Ft ) 是已知最具传染性的病原体之一。 Ft毒力由转录调节因子的独特组合控制:MglA-SspA 异二聚体、PigR 和应激信号 ppGpp。 MglA-SspA 与 σ 70相关 RNAP 全酶 (RNAPσ 70 ) 组装,形成毒力特异性聚合酶。这些因子激活弗朗西斯菌致病岛(FPI)基因表达,这是毒力所必需的,但其机制尚不清楚。在这里,我们报告了Ft RNAPσ 70 -启动子-DNA、 Ft RNAPσ 70 -(MglA-SspA)-启动子 DNA 和Ft RNAPσ 70 -(MglA-SspA)-ppGpp-PigR-启动子 DNA 冷冻电镜结构。结构和遗传分析表明 MglA-SspA 促进 σ 70与 DNA 结合,从而调节毒力和毒力增强基因。我们的大肠杆菌RNAPσ 70-同源二聚体Ec SspA 结构表明这是一种通用的 SspA 转录调控机制。引人注目的是,我们的Ft RNAPσ 70 -(MglA-SspA)-ppGpp-PigR-DNA 结构揭示了 ppGpp 与 MglA-SspA 的结合将 PigR 束缚到启动子上。 PigR 反过来将Ft RNAP αCTD 招募到 DNA UP 元件上。因此,这些研究揭示了Ft发病机制的独特机制,涉及毒力特异性 RNAP,该 RNAP 采用两种基于 (MglA-SspA) 的策略来激活毒力基因。











































 京公网安备 11010802027423号
京公网安备 11010802027423号