Journal of Molecular Biology ( IF 4.7 ) Pub Date : 2020-11-19 , DOI: 10.1016/j.jmb.2020.11.013 Salman Shahid 1 , Mingming Gao 1 , D Travis Gallagher 2 , Edwin Pozharski 3 , Robert G Brinson 2 , Zhen-Yong Keck 4 , Steven K H Foung 4 , Thomas R Fuerst 1 , Roy A Mariuzza 1
|
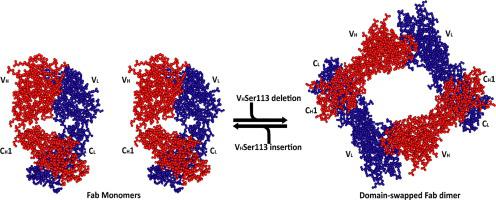
We determined the crystal structure to 1.8 Å resolution of the Fab fragment of an affinity-matured human monoclonal antibody (HC84.26.5D) that recognizes the E2 envelope glycoprotein of hepatitis C virus (HCV). Unlike conventional Fabs, which are monovalent monomers, Fab HC84.26.5D assembles into a bivalent domain-swapped dimer in which the two VL/VH modules are separated by ~25 Å. In solution, Fab HC84.26.5D exists predominantly as a dimer (~80%) in equilibrium with the monomeric form of the Fab (~20%). Dimerization is mediated entirely by deletion of a single residue, VHSer113 (Kabat numbering), in the elbow region linking the VH and CH1 domains. In agreement with the crystal structure, dimeric Fab HC84.26.5D is able to bind two HCV E2 molecules in solution. This is only the second example of a domain-swapped Fab dimer from among >3000 Fab crystal structures determined to date. Moreover, the architecture of the doughnut-shaped Fab HC84.26.5D dimer is completely different from that of the previously reported Fab 2G12 dimer. We demonstrate that the highly identifiable shape of dimeric Fab HC84.26.5D makes it useful as a fiducial marker for single-particle cryoEM analysis of HCV E2. Bivalent domain-swapped Fab dimers engineered on the basis of HC84.26.5D may also serve as a means of doubling the effective size of conventional Fab–protein complexes for cryoEM.
中文翻译:

二价抗体 Fab 片段的晶体结构
我们将亲和力成熟的人单克隆抗体 (HC84.26.5D) 的 Fab 片段的晶体结构确定为 1.8 Å 分辨率,该抗体可识别丙型肝炎病毒 (HCV) 的 E2 包膜糖蛋白。与作为单价单体的传统 Fab 不同,Fab HC84.26.5D 组装成二价结构域交换二聚体,其中两个 V L /V H模块相隔约 25 Å。在溶液中,Fab HC84.26.5D 主要作为二聚体 (~80%) 存在,与 Fab 的单体形式 (~20%) 处于平衡状态。二聚化完全由单个残基的缺失介导,V H Ser113(Kabat 编号),位于连接 V H和 C H的肘部区域1 个域。与晶体结构一致,二聚体 Fab HC84.26.5D 能够结合溶液中的两个 HCV E2 分子。这只是迄今为止确定的 > 3000 个 Fab 晶体结构中域交换 Fab 二聚体的第二个例子。此外,环形 Fab HC84.26.5D 二聚体的结构与之前报道的 Fab 2G12 二聚体的结构完全不同。我们证明二聚体 Fab HC84.26.5D 的高度可识别形状使其可用作 HCV E2 单粒子冷冻电镜分析的基准标记。在 HC84.26.5D 的基础上设计的二价结构域交换 Fab 二聚体也可以作为一种方法,将用于 cryoEM 的传统 Fab-蛋白质复合物的有效大小加倍。











































 京公网安备 11010802027423号
京公网安备 11010802027423号