当前位置:
X-MOL 学术
›
Metallomics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Lanthanum chloride causes blood–brain barrier disruption through intracellular calcium-mediated RhoA/Rho kinase signaling and myosin light chain kinase
Metallomics ( IF 2.9 ) Pub Date : 2020-11-18 , DOI: 10.1039/d0mt00187b Jie Wu 1 , Jinghua Yang , Miao Yu , Wenchang Sun , Yarao Han , Xiaobo Lu , Cuihong Jin , Shengwen Wu , Yuan Cai
Metallomics ( IF 2.9 ) Pub Date : 2020-11-18 , DOI: 10.1039/d0mt00187b Jie Wu 1 , Jinghua Yang , Miao Yu , Wenchang Sun , Yarao Han , Xiaobo Lu , Cuihong Jin , Shengwen Wu , Yuan Cai
Affiliation
|
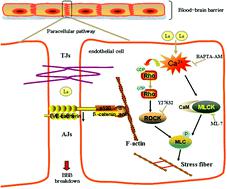
Rare earth elements (REEs) have caused bioaccumulation and adverse health effects attributed to extensive application. The penetrability of REEs across the blood–brain barrier (BBB) contributes to their neurotoxicity process, but potential mechanisms affecting BBB integrity are still obscure. The present study was designed to investigate the effects of lanthanum on BBB adheren junctions and the actin cytoskeleton in vitro using bEnd.3 cells. After lanthanum chloride (LaCl3, 0.125, 0.25 and 0.5 mM) treatment, cytotoxicity against bEnd.3 cells was observed accompanied by increased intracellular Ca2+. Higher paracellular permeability presented as decreased TEER (transendothelial electrical resistance) and increased HRP (horse radish peroxidase) permeation, and simultaneously reduced VE-cadherin expression and F-actin stress fiber formation caused by LaCl3 were reversed by inhibition of ROCK (Rho-kinase) and MLCK (myosin light chain kinase) using inhibitor Y27632 (10 μM) and ML-7 (10 μM). Moreover, chelating overloaded intracellular Ca2+ by BAPTA-AM (25 μM) remarkably abrogated RhoA/ROCK and MLCK activation and downstream phosphorylation of MYPT1 (myosin phosphatase target subunit 1) and MLC2 (myosin light chain 2), therefore alleviating LaCl3-induced BBB disruption and dysfunction. In conclusion, this study indicated that lanthanum caused endothelial barrier hyperpermeability accompanied by loss of VE-cadherin and rearrangement of the actin cytoskeleton though intracellular Ca2+-mediated RhoA/ROCK and MLCK pathways.
中文翻译:

氯化镧通过细胞内钙介导的 RhoA/Rho 激酶信号和肌球蛋白轻链激酶导致血脑屏障破坏
稀土元素 (REE) 由于广泛应用而导致生物蓄积和不利健康影响。REE 穿过血脑屏障 (BBB) 的渗透性有助于其神经毒性过程,但影响 BBB 完整性的潜在机制仍不清楚。本研究旨在使用 bEnd.3 细胞在体外研究镧对 BBB 粘附连接和肌动蛋白细胞骨架的影响。在氯化镧 (LaCl 3 , 0.125, 0.25 和 0.5 mM) 处理后,观察到对 bEnd.3 细胞的细胞毒性伴随着细胞内 Ca 2+的增加. 较高的细胞旁通透性表现为 TEER(跨内皮电阻)降低和 HRP(辣根过氧化物酶)渗透增加,同时由 LaCl 3引起的 VE-钙粘蛋白表达和 F-肌动蛋白应力纤维形成被抑制 ROCK(Rho-激酶)逆转) 和 MLCK(肌球蛋白轻链激酶),使用抑制剂 Y27632 (10 μM) 和 ML-7 (10 μM)。此外,通过 BAPTA-AM (25 μM)螯合超载的细胞内 Ca 2+显着消除了 RhoA/ROCK 和 MLCK 的活化以及 MYPT1(肌球蛋白磷酸酶靶标亚基 1)和 MLC2(肌球蛋白轻链 2)的下游磷酸化,因此减轻了 LaCl 3- 诱导 BBB 中断和功能障碍。总之,该研究表明,镧通过细胞内 Ca 2+介导的 RhoA/ROCK 和 MLCK 途径引起内皮屏障高渗透性,伴随着 VE-钙粘蛋白的丧失和肌动蛋白细胞骨架的重排。
更新日期:2021-01-06
中文翻译:

氯化镧通过细胞内钙介导的 RhoA/Rho 激酶信号和肌球蛋白轻链激酶导致血脑屏障破坏
稀土元素 (REE) 由于广泛应用而导致生物蓄积和不利健康影响。REE 穿过血脑屏障 (BBB) 的渗透性有助于其神经毒性过程,但影响 BBB 完整性的潜在机制仍不清楚。本研究旨在使用 bEnd.3 细胞在体外研究镧对 BBB 粘附连接和肌动蛋白细胞骨架的影响。在氯化镧 (LaCl 3 , 0.125, 0.25 和 0.5 mM) 处理后,观察到对 bEnd.3 细胞的细胞毒性伴随着细胞内 Ca 2+的增加. 较高的细胞旁通透性表现为 TEER(跨内皮电阻)降低和 HRP(辣根过氧化物酶)渗透增加,同时由 LaCl 3引起的 VE-钙粘蛋白表达和 F-肌动蛋白应力纤维形成被抑制 ROCK(Rho-激酶)逆转) 和 MLCK(肌球蛋白轻链激酶),使用抑制剂 Y27632 (10 μM) 和 ML-7 (10 μM)。此外,通过 BAPTA-AM (25 μM)螯合超载的细胞内 Ca 2+显着消除了 RhoA/ROCK 和 MLCK 的活化以及 MYPT1(肌球蛋白磷酸酶靶标亚基 1)和 MLC2(肌球蛋白轻链 2)的下游磷酸化,因此减轻了 LaCl 3- 诱导 BBB 中断和功能障碍。总之,该研究表明,镧通过细胞内 Ca 2+介导的 RhoA/ROCK 和 MLCK 途径引起内皮屏障高渗透性,伴随着 VE-钙粘蛋白的丧失和肌动蛋白细胞骨架的重排。











































 京公网安备 11010802027423号
京公网安备 11010802027423号