当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unique Hydrogen Bonding of Adenine with the Oxidatively Damaged Base 8-Oxoguanine Enables Specific Recognition and Repair by DNA Glycosylase MutY
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-11-17 , DOI: 10.1021/jacs.0c06767 Chandrima Majumdar 1 , Paige L McKibbin 1 , Allison E Krajewski 2 , Amelia H Manlove 1 , Jeehiun K Lee 2 , Sheila S David 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-11-17 , DOI: 10.1021/jacs.0c06767 Chandrima Majumdar 1 , Paige L McKibbin 1 , Allison E Krajewski 2 , Amelia H Manlove 1 , Jeehiun K Lee 2 , Sheila S David 1
Affiliation
|
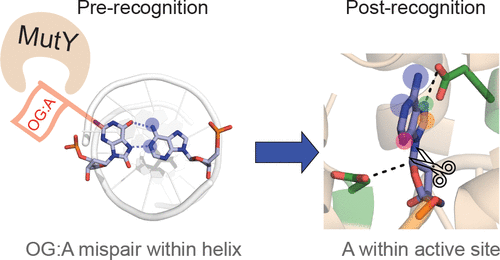
The DNA glycosylase MutY prevents deleterious mutations resulting from guanine oxidation by recognition and removal of adenine (A) misincorporated opposite 8-oxo-7,8-dihydroguanine (OG). Correct identification of OG:A is crucial to prevent improper and detrimental MutY-mediatedadenine excision from G:A or T:A base pairs. Here we present a structure-activity relationship (SAR) study using analogues of A to probe the basis for OG:A specificity of MutY. We correlate observed in vitro MutY activity on A analogue substrates with their experimental and calculated acidities to provide mechanistic insight into the factors influencing MutY base excision efficiency. These data show that H-bonding and electrostatic interactions of the base within the MutY active site modulate the lability of the N-glycosidic bond. A analogues that were not excised from duplex DNA as efficiently as predicted by calculations provided insight into other required structural features, such as steric fit and H-bonding within the active site for proper alignment with MutY catalytic residues. We also determined MutY-mediated repair of A analogues paired with OG within the context of a DNA plasmid in bacteria. Remarkably, the magnitudes of decreased in vitro MutY excision rates with different A analogue duplexes do not correlate with the impact on overall MutY-mediated repair. The feature that most strongly correlated with facile cellular repair was the ability of the A analogues to H-bond with the Hoogsteen face of OG. Notably, base pairing of A with OG uniquely positions the 2-amino group of OG in the major groove and provides a means to indirectly select only these inappropriately placed adenines for excision. This highlights the importance of OG lesion detection for efficient MutY-mediated cellular repair. The A analogue SARs also highlight the types of modifications tolerated by MutY and will guide the development of specific probes and inhibitors of MutY.
中文翻译:

腺嘌呤与氧化损伤碱基 8-氧鸟嘌呤的独特氢键可通过 DNA 糖基化酶 MutY 进行特异性识别和修复
DNA 糖基化酶 MutY 通过识别和去除与 8-oxo-7,8-二氢鸟嘌呤 (OG) 相对的腺嘌呤 (A) 来防止鸟嘌呤氧化导致的有害突变。OG:A 的正确识别对于防止从 G:A 或 T:A 碱基对中不正确和有害的 MutY 介导的腺嘌呤切除至关重要。在这里,我们提出了一项结构-活性关系 (SAR) 研究,使用 A 的类似物来探索 OG:A MutY 的特异性的基础。我们将在 A 类似物底物上观察到的体外 MutY 活性与其实验和计算的酸度相关联,以提供对影响 MutY 碱基切除效率的因素的机制洞察。这些数据表明,MutY 活性位点内碱基的氢键和静电相互作用调节了 N-糖苷键的不稳定性。没有像计算预测的那样有效地从双链 DNA 中切除的类似物提供了对其他所需结构特征的洞察,例如活性位点内的空间拟合和 H 键合,以便与 MutY 催化残基正确对齐。我们还在细菌中的 DNA 质粒的背景下确定了 MutY 介导的 A 类似物与 OG 配对的修复。值得注意的是,不同 A 模拟双链体的体外 MutY 切除率降低的幅度与对整体 MutY 介导的修复的影响无关。与轻松的细胞修复最密切相关的特征是 A 类似物与 OG 的 Hoogsteen 面形成 H 键的能力。尤其,A 与 OG 的碱基配对将 OG 的 2-氨基独特地定位在大沟中,并提供了一种仅间接选择这些不适当放置的腺嘌呤进行切除的方法。这突出了 OG 病变检测对于高效 MutY 介导的细胞修复的重要性。A 类似物 SAR 还突出了 MutY 耐受的修饰类型,并将指导 MutY 的特定探针和抑制剂的开发。
更新日期:2020-11-17
中文翻译:

腺嘌呤与氧化损伤碱基 8-氧鸟嘌呤的独特氢键可通过 DNA 糖基化酶 MutY 进行特异性识别和修复
DNA 糖基化酶 MutY 通过识别和去除与 8-oxo-7,8-二氢鸟嘌呤 (OG) 相对的腺嘌呤 (A) 来防止鸟嘌呤氧化导致的有害突变。OG:A 的正确识别对于防止从 G:A 或 T:A 碱基对中不正确和有害的 MutY 介导的腺嘌呤切除至关重要。在这里,我们提出了一项结构-活性关系 (SAR) 研究,使用 A 的类似物来探索 OG:A MutY 的特异性的基础。我们将在 A 类似物底物上观察到的体外 MutY 活性与其实验和计算的酸度相关联,以提供对影响 MutY 碱基切除效率的因素的机制洞察。这些数据表明,MutY 活性位点内碱基的氢键和静电相互作用调节了 N-糖苷键的不稳定性。没有像计算预测的那样有效地从双链 DNA 中切除的类似物提供了对其他所需结构特征的洞察,例如活性位点内的空间拟合和 H 键合,以便与 MutY 催化残基正确对齐。我们还在细菌中的 DNA 质粒的背景下确定了 MutY 介导的 A 类似物与 OG 配对的修复。值得注意的是,不同 A 模拟双链体的体外 MutY 切除率降低的幅度与对整体 MutY 介导的修复的影响无关。与轻松的细胞修复最密切相关的特征是 A 类似物与 OG 的 Hoogsteen 面形成 H 键的能力。尤其,A 与 OG 的碱基配对将 OG 的 2-氨基独特地定位在大沟中,并提供了一种仅间接选择这些不适当放置的腺嘌呤进行切除的方法。这突出了 OG 病变检测对于高效 MutY 介导的细胞修复的重要性。A 类似物 SAR 还突出了 MutY 耐受的修饰类型,并将指导 MutY 的特定探针和抑制剂的开发。











































 京公网安备 11010802027423号
京公网安备 11010802027423号