当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Reactivity, Formation, and Solubility of Polyoxometalates Probed by Calorimetry
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2020-11-17 , DOI: 10.1021/jacs.0c10133 Hrafn Traustason 1 , Nicola L. Bell 2 , Kiana Caranto 3 , David C. Auld 2 , David T. Lockey 2 , Alex Kokot 3 , Jennifer E.S. Szymanowski 3 , Leroy Cronin 2 , Peter C. Burns 1, 3
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2020-11-17 , DOI: 10.1021/jacs.0c10133 Hrafn Traustason 1 , Nicola L. Bell 2 , Kiana Caranto 3 , David C. Auld 2 , David T. Lockey 2 , Alex Kokot 3 , Jennifer E.S. Szymanowski 3 , Leroy Cronin 2 , Peter C. Burns 1, 3
Affiliation
|
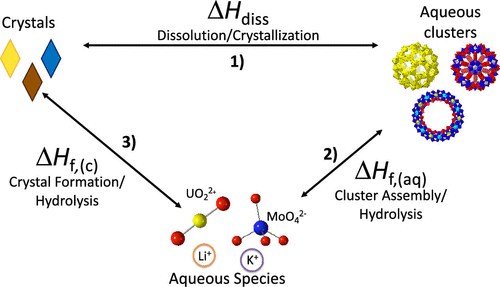
Room temperature calorimetry methods were developed to describe the energy landscapes of six polyoxometalates (POMs), Li-U24, Li-U28, K-U28, Li/K-U60, Mo132, and Mo154, in terms of three components: enthalpy of dissolution (ΔHdiss), enthalpy of formation of aqueous POMs (ΔHf,(aq)), and enthalpy of formation of POM crystals (ΔHf,(c)). ΔHdiss is controlled by a combination of cation solvation enthalpy and the favorability of cation interactions with binding sites on the POM. In the case of the four uranyl peroxide POMs studied, clusters with hydroxide bridges have lower ΔHf,(aq) and are more stable than those containing only peroxide bridges. In general for POMs, the combination of calorimetric results and synthetic observations suggest that spherical topologies may be more stable than wheel-like clusters, and ΔHf,(aq) can be accurately estimated using only ΔHf,(c) values owing to the dominance of the clusters in determining the energetics of POM crystals.
中文翻译:

量热法探测多金属氧酸盐的反应性、形成和溶解度
开发了室温量热法来描述六种多金属氧酸盐 (POM)、Li-U24、Li-U28、K-U28、Li/K-U60、Mo132 和 Mo154 的能量图谱,根据三个组成部分:溶解焓(ΔHdiss)、水性 POM 的形成焓 (ΔHf,(aq)) 和 POM 晶体的形成焓 (ΔHf,(c))。ΔHdiss 受阳离子溶剂化焓和阳离子与 POM 上结合位点相互作用的有利性的组合控制。在研究的四种过氧化铀 POM 的情况下,具有氢氧化物桥的簇具有较低的 ΔHf,(aq) 并且比仅包含过氧化物桥的簇更稳定。一般来说,对于 POM,量热结果和综合观察的结合表明球形拓扑可能比轮状簇更稳定,并且 ΔHf,
更新日期:2020-11-17
中文翻译:

量热法探测多金属氧酸盐的反应性、形成和溶解度
开发了室温量热法来描述六种多金属氧酸盐 (POM)、Li-U24、Li-U28、K-U28、Li/K-U60、Mo132 和 Mo154 的能量图谱,根据三个组成部分:溶解焓(ΔHdiss)、水性 POM 的形成焓 (ΔHf,(aq)) 和 POM 晶体的形成焓 (ΔHf,(c))。ΔHdiss 受阳离子溶剂化焓和阳离子与 POM 上结合位点相互作用的有利性的组合控制。在研究的四种过氧化铀 POM 的情况下,具有氢氧化物桥的簇具有较低的 ΔHf,(aq) 并且比仅包含过氧化物桥的簇更稳定。一般来说,对于 POM,量热结果和综合观察的结合表明球形拓扑可能比轮状簇更稳定,并且 ΔHf,



























 京公网安备 11010802027423号
京公网安备 11010802027423号