当前位置:
X-MOL 学术
›
Chem. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Microkinetic Modeling: A Tool for Rational Catalyst Design
Chemical Reviews ( IF 62.1 ) Pub Date : 2020-11-18 , DOI: 10.1021/acs.chemrev.0c00394 Ali Hussain Motagamwala 1 , James A. Dumesic 1
Chemical Reviews ( IF 62.1 ) Pub Date : 2020-11-18 , DOI: 10.1021/acs.chemrev.0c00394 Ali Hussain Motagamwala 1 , James A. Dumesic 1
Affiliation
|
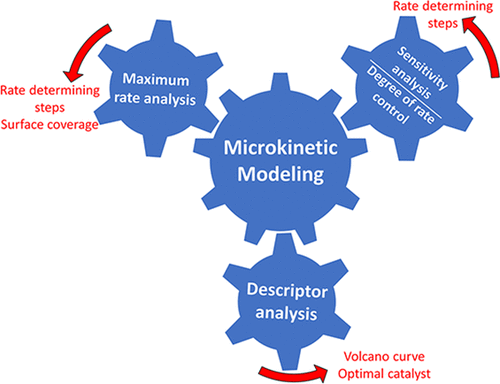
The design of heterogeneous catalysts relies on understanding the fundamental surface kinetics that controls catalyst performance, and microkinetic modeling is a tool that can help the researcher in streamlining the process of catalyst design. Microkinetic modeling is used to identify critical reaction intermediates and rate-determining elementary reactions, thereby providing vital information for designing an improved catalyst. In this review, we summarize general procedures for developing microkinetic models using reaction kinetics parameters obtained from experimental data, theoretical correlations, and quantum chemical calculations. We examine the methods required to ensure the thermodynamic consistency of the microkinetic model. We describe procedures required for parameter adjustments to account for the heterogeneity of the catalyst and the inherent errors in parameter estimation. We discuss the analysis of microkinetic models to determine the rate-determining reactions using the degree of rate control and reversibility of each elementary reaction. We introduce incorporation of Brønsted–Evans–Polanyi relations and scaling relations in microkinetic models and the effects of these relations on catalytic performance and formation of volcano curves are discussed. We review the analysis of reaction schemes in terms of the maximum rate of elementary reactions, and we outline a procedure to identify kinetically significant transition states and adsorbed intermediates. We explore the application of generalized rate expressions for the prediction of optimal binding energies of important surface intermediates and to estimate the extent of potential rate improvement. We also explore the application of microkinetic modeling in homogeneous catalysis, electro-catalysis, and transient reaction kinetics. We conclude by highlighting the challenges and opportunities in the application of microkinetic modeling for catalyst design.
中文翻译:

微观动力学建模:合理催化剂设计的工具
非均相催化剂的设计依赖于对控制催化剂性能的基本表面动力学的理解,而微动力学建模是可以帮助研究人员简化催化剂设计过程的工具。微动力学模型用于识别关键的反应中间体和决定基本反应的速率,从而为设计改进的催化剂提供重要信息。在这篇综述中,我们总结了使用从实验数据,理论相关性和量子化学计算获得的反应动力学参数开发微动力学模型的一般程序。我们研究了确保微动力学模型热力学一致性所需的方法。我们描述了参数调整所需的程序,以解决催化剂的异质性和参数估计中的固有误差。我们讨论了使用速率控制和每个基本反应的可逆性来确定速率确定反应的微观动力学模型的分析。我们介绍了在微动力学模型中纳入Brønsted–Evans–Polanyi关系和比例关系,并讨论了这些关系对催化性能和火山曲线形成的影响。我们根据基本反应的最大速率回顾了反应方案的分析,并概述了确定动力学上重要的过渡态和吸附的中间体的程序。我们探索广义速率表达式在重要表面中间体的最佳结合能预测中的应用,并估计潜在速率改善的程度。我们还探索了微动力学模型在均相催化,电催化和瞬态反应动力学中的应用。最后,我们着重介绍了微动力学建模在催化剂设计中的应用所面临的挑战和机遇。
更新日期:2021-01-27
中文翻译:

微观动力学建模:合理催化剂设计的工具
非均相催化剂的设计依赖于对控制催化剂性能的基本表面动力学的理解,而微动力学建模是可以帮助研究人员简化催化剂设计过程的工具。微动力学模型用于识别关键的反应中间体和决定基本反应的速率,从而为设计改进的催化剂提供重要信息。在这篇综述中,我们总结了使用从实验数据,理论相关性和量子化学计算获得的反应动力学参数开发微动力学模型的一般程序。我们研究了确保微动力学模型热力学一致性所需的方法。我们描述了参数调整所需的程序,以解决催化剂的异质性和参数估计中的固有误差。我们讨论了使用速率控制和每个基本反应的可逆性来确定速率确定反应的微观动力学模型的分析。我们介绍了在微动力学模型中纳入Brønsted–Evans–Polanyi关系和比例关系,并讨论了这些关系对催化性能和火山曲线形成的影响。我们根据基本反应的最大速率回顾了反应方案的分析,并概述了确定动力学上重要的过渡态和吸附的中间体的程序。我们探索广义速率表达式在重要表面中间体的最佳结合能预测中的应用,并估计潜在速率改善的程度。我们还探索了微动力学模型在均相催化,电催化和瞬态反应动力学中的应用。最后,我们着重介绍了微动力学建模在催化剂设计中的应用所面临的挑战和机遇。



























 京公网安备 11010802027423号
京公网安备 11010802027423号