当前位置:
X-MOL 学术
›
Chem. Rec.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Applications of Transition‐Metal‐Catalyzed Asymmetric Allylic Substitution in Total Synthesis of Natural Products: An Update
The Chemical Record ( IF 7.0 ) Pub Date : 2020-11-18 , DOI: 10.1002/tcr.202000086 Leyla Mohammadkhani 1 , Majid M Heravi 1
The Chemical Record ( IF 7.0 ) Pub Date : 2020-11-18 , DOI: 10.1002/tcr.202000086 Leyla Mohammadkhani 1 , Majid M Heravi 1
Affiliation
|
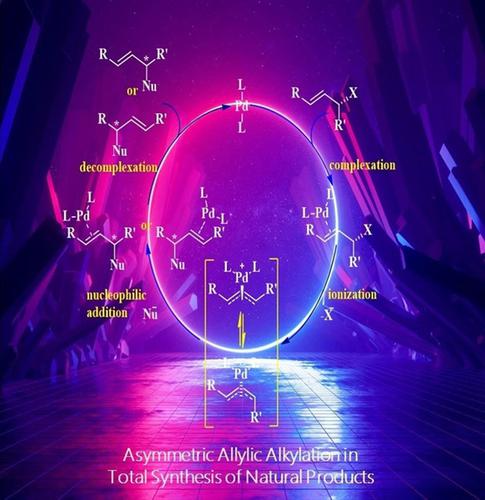
Metal‐catalyzed asymmetric allylic substitution (AAS) reaction is one of the most synthetically useful reactions catalyzed by metal complexes for the formation of carbon‐carbon and carbon‐heteroatom bonds. It comprises the substitution of allylic substrates with a wide range of nucleophiles or SN2′‐type allylic substitution, which results in the formation of the above‐mentioned bonds with high levels of enantioselective induction. AAS reaction tolerates a broad range of functional groups, thus has been successfully applied in the asymmetric synthesis of a wide range of optically pure compounds. This reaction has been extensively used in the total synthesis of several complex molecules, especially natural products. In this review, we try to highlight the applications of metal (Pd, Ir, Mo, or Cu)‐catalyzed AAS reaction in the total synthesis of the biologically active natural products, as a key step, updating the subject from 2003 till date.
中文翻译:

过渡金属催化不对称烯丙基取代在天然产物全合成中的应用
金属催化的不对称烯丙基取代(AAS)反应是金属络合物催化形成碳-碳和碳-杂原子键的最有用的反应之一。它包括用多种亲核试剂或S N取代烯丙基底物2'型烯丙基取代,导致上述键的形成,具有高水平的对映选择性诱导。AAS反应可耐受各种官能团,因此已成功地用于各种光学纯化合物的不对称合成中。该反应已广泛用于几种复杂分子,特别是天然产物的总合成中。在这篇综述中,我们试图强调金属(Pd,Ir,Mo或Cu)催化的AAS反应在生物活性天然产物的总合成中的应用,这是关键步骤,从2003年至今一直更新该主题。
更新日期:2021-01-21
中文翻译:

过渡金属催化不对称烯丙基取代在天然产物全合成中的应用
金属催化的不对称烯丙基取代(AAS)反应是金属络合物催化形成碳-碳和碳-杂原子键的最有用的反应之一。它包括用多种亲核试剂或S N取代烯丙基底物2'型烯丙基取代,导致上述键的形成,具有高水平的对映选择性诱导。AAS反应可耐受各种官能团,因此已成功地用于各种光学纯化合物的不对称合成中。该反应已广泛用于几种复杂分子,特别是天然产物的总合成中。在这篇综述中,我们试图强调金属(Pd,Ir,Mo或Cu)催化的AAS反应在生物活性天然产物的总合成中的应用,这是关键步骤,从2003年至今一直更新该主题。











































 京公网安备 11010802027423号
京公网安备 11010802027423号