当前位置:
X-MOL 学术
›
J. Leukoc. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Inducing regulated necrosis and shifting macrophage polarization with anti-EMMPRIN antibody (161-pAb) and complement factors
Journal of Leukocyte Biology ( IF 3.6 ) Pub Date : 2020-11-17 , DOI: 10.1002/jlb.3a0520-333r Nizar Hijaze 1 , Max Ledersnaider 2 , Elina Simanovich 2 , Sameer Kassem 1, 3 , Michal A Rahat 2, 3
Journal of Leukocyte Biology ( IF 3.6 ) Pub Date : 2020-11-17 , DOI: 10.1002/jlb.3a0520-333r Nizar Hijaze 1 , Max Ledersnaider 2 , Elina Simanovich 2 , Sameer Kassem 1, 3 , Michal A Rahat 2, 3
Affiliation
|
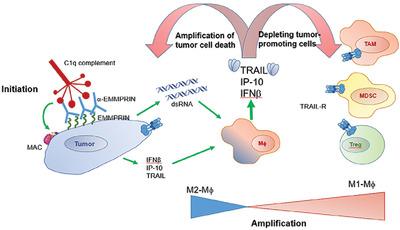
Treatment of solid tumors is often hindered by an immunosuppressive tumor microenvironment (TME) that prevents effector immune cells from eradicating tumor cells and promotes tumor progression, angiogenesis, and metastasis. Therefore, targeting components of the TME to restore the ability of immune cells to drive anti-tumoral responses has become an important goal. One option is to induce an immunogenic cell death (ICD) of tumor cells that would trigger an adaptive anti-tumoral immune response. Here we show that incubating mouse renal cell carcinoma (RENCA) and colon carcinoma cell lines with an anti-extracellular matrix metalloproteinase inducer polyclonal antibody (161-pAb) together with complement factors can induce cell death that inhibits caspase-8 activity and enhances the phosphorylation of receptor-interacting protein kinase 3 (RIPK3) and mixed-lineage kinase-like domain (MLKL). This regulated necrotic death releases high levels of dsRNA molecules to the conditioned medium (CM) relative to the necrotic death of tumor cells induced by H2O2 or the apoptotic death induced by etoposide. RAW 264.7 macrophages incubated with the CM derived from these dying cells markedly enhanced the secretion of IFNβ, and enhanced their cytotoxicity. Furthermore, degradation of the dsRNA in the CM abolished the ability of RAW 264.7 macrophages to secrete IFNβ, IFNγ-induced protein 10 (IP-10), and TRAIL. When mice bearing RENCA tumors were immunized with the 161-pAb, their lysates displayed elevated levels of phosphorylated RIPK3 and MLKL, as well as increased concentrations of dsRNA, IFNβ, IP-10, and TRAIL. This shows that an antigen-targeted therapy using an antibody and complement factors that triggers ICD can shift the mode of macrophage activation by triggering regulated necrotic death of tumor cells.
中文翻译:

使用抗 EMMPRIN 抗体 (161-pAb) 和补体因子诱导调节性坏死并改变巨噬细胞极化
实体瘤的治疗常常受到免疫抑制性肿瘤微环境(TME)的阻碍,该微环境会阻止效应免疫细胞根除肿瘤细胞并促进肿瘤进展、血管生成和转移。因此,靶向TME的成分以恢复免疫细胞驱动抗肿瘤反应的能力已成为一个重要目标。一种选择是诱导肿瘤细胞的免疫原性细胞死亡(ICD),从而触发适应性抗肿瘤免疫反应。在这里,我们表明,用抗细胞外基质金属蛋白酶诱导剂多克隆抗体 (161-pAb) 与补体因子一起孵育小鼠肾细胞癌 (RENCA) 和结肠癌细胞系,可以诱导细胞死亡,从而抑制 caspase-8 活性并增强磷酸化受体相互作用蛋白激酶 3 (RIPK3) 和混合谱系激酶样结构域 (MLKL)。相对于H 2 O 2诱导的肿瘤细胞坏死性死亡或依托泊苷诱导的细胞凋亡,这种受调节的坏死性死亡向条件培养基(CM)释放高水平的dsRNA分子。 RAW 264.7 巨噬细胞与来自这些垂死细胞的 CM 一起孵育,显着增强了 IFNβ 的分泌,并增强了其细胞毒性。此外,CM 中 dsRNA 的降解消除了 RAW 264.7 巨噬细胞分泌 IFNβ、IFNγ 诱导蛋白 10 (IP-10) 和 TRAIL 的能力。当携带 RENCA 肿瘤的小鼠接受 161-pAb 免疫时,其裂解物显示磷酸化 RIPK3 和 MLKL 水平升高,以及 dsRNA、IFNβ、IP-10 和 TRAIL 浓度升高。 这表明使用触发 ICD 的抗体和补体因子的抗原靶向治疗可以通过触发肿瘤细胞的受调节坏死性死亡来改变巨噬细胞激活模式。
更新日期:2020-11-17
中文翻译:

使用抗 EMMPRIN 抗体 (161-pAb) 和补体因子诱导调节性坏死并改变巨噬细胞极化
实体瘤的治疗常常受到免疫抑制性肿瘤微环境(TME)的阻碍,该微环境会阻止效应免疫细胞根除肿瘤细胞并促进肿瘤进展、血管生成和转移。因此,靶向TME的成分以恢复免疫细胞驱动抗肿瘤反应的能力已成为一个重要目标。一种选择是诱导肿瘤细胞的免疫原性细胞死亡(ICD),从而触发适应性抗肿瘤免疫反应。在这里,我们表明,用抗细胞外基质金属蛋白酶诱导剂多克隆抗体 (161-pAb) 与补体因子一起孵育小鼠肾细胞癌 (RENCA) 和结肠癌细胞系,可以诱导细胞死亡,从而抑制 caspase-8 活性并增强磷酸化受体相互作用蛋白激酶 3 (RIPK3) 和混合谱系激酶样结构域 (MLKL)。相对于H 2 O 2诱导的肿瘤细胞坏死性死亡或依托泊苷诱导的细胞凋亡,这种受调节的坏死性死亡向条件培养基(CM)释放高水平的dsRNA分子。 RAW 264.7 巨噬细胞与来自这些垂死细胞的 CM 一起孵育,显着增强了 IFNβ 的分泌,并增强了其细胞毒性。此外,CM 中 dsRNA 的降解消除了 RAW 264.7 巨噬细胞分泌 IFNβ、IFNγ 诱导蛋白 10 (IP-10) 和 TRAIL 的能力。当携带 RENCA 肿瘤的小鼠接受 161-pAb 免疫时,其裂解物显示磷酸化 RIPK3 和 MLKL 水平升高,以及 dsRNA、IFNβ、IP-10 和 TRAIL 浓度升高。 这表明使用触发 ICD 的抗体和补体因子的抗原靶向治疗可以通过触发肿瘤细胞的受调节坏死性死亡来改变巨噬细胞激活模式。











































 京公网安备 11010802027423号
京公网安备 11010802027423号