Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
PARP1 and PRC2 double deficiency promotes BRCA‐proficient breast cancer growth by modification of the tumor microenvironment
The FEBS Journal ( IF 5.5 ) Pub Date : 2020-11-18 , DOI: 10.1111/febs.15636 A-Yeong Yang 1 , Eun-Bee Choi 2, 3 , Mi So Park 1 , Seon-Kyu Kim 4 , Min-Seok Park 1 , Mi-Young Kim 1, 5
The FEBS Journal ( IF 5.5 ) Pub Date : 2020-11-18 , DOI: 10.1111/febs.15636 A-Yeong Yang 1 , Eun-Bee Choi 2, 3 , Mi So Park 1 , Seon-Kyu Kim 4 , Min-Seok Park 1 , Mi-Young Kim 1, 5
Affiliation
|
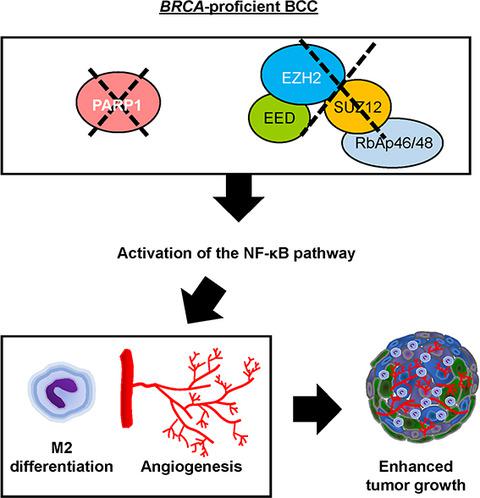
Poly (ADP‐ribose) polymerase 1 (PARP1) and polycomb‐repressive complex 2 (PRC2) are each known for their individual roles in cancer, but their cooperative roles have only been studied in the DNA damage repair process in the context of BRCA‐mutant cancers. Here, we show that simultaneous inhibition of PARP1 and PRC2 in the MDA‐MB‐231 BRCA‐proficient triple‐negative breast cancer (TNBC) cell line leads to a synthetic viability independent of the mechanisms of DNA damage repair. Specifically, we find that either genetic depletion or pharmacological inhibition of both PARP1 and PRC2 can accelerate tumor growth rate. We attribute this to modifications in the tumor microenvironment (TME) that are induced by double‐depleted breast cancer cells, such as promoting intratumoral angiogenesis and increasing the proportion of tumor‐promoting type 2 (M2) macrophages. These changes subsequently inhibit cell death and promote proliferation. Mechanistically, we find that PARP1 and PRC2 double depletion induces not only a basal activation of the NF‐κB pathway but also a maximal activation of NF‐κB within the TME in response to external stimuli such as hypoxia and the presence of macrophages. In summary, our study reveals an unprecedented synthetic viable interaction between PARP1 and PRC2 in BRCA‐proficient TNBC and identifies NF‐κB as the downstream mediator.
中文翻译:

PARP1和PRC2双重缺陷通过改变肿瘤微环境促进BRCA熟练的乳腺癌生长
聚(ADP-核糖)聚合酶-1(PARP1)和梳压抑复合物2(PRC2)各自已知的用于癌症的个体的角色,但他们的合作作用只在DNA损伤修复过程被研究的背景下BRCA -突变型癌症。在这里,我们显示,在MDA-MB-231 BRCA精通的三阴性乳腺癌(TNBC)细胞系中同时抑制PARP1和PRC2可以导致合成活力,而与DNA损伤修复机制无关。具体来说,我们发现PARP1和PRC2的遗传损耗或药理抑制作用可以加快肿瘤的生长速度。我们将其归因于双重耗尽的乳腺癌细胞在肿瘤微环境(TME)中的修饰,例如促进肿瘤内血管生成和增加肿瘤促进2型(M2)巨噬细胞的比例。这些变化随后抑制细胞死亡并促进增殖。从机理上讲,我们发现PARP1和PRC2的双耗竭不仅会诱导TME中NF-κB途径的基础活化,而且还会在外部刺激(如缺氧和巨噬细胞的存在)的反应中最大程度地激活NF-κB的活化。总而言之,我们的研究揭示了BRCA中PARP1和PRC2之间前所未有的合成可行的相互作用精通TNBC,并将NF-κB识别为下游介体。
更新日期:2020-11-18
中文翻译:

PARP1和PRC2双重缺陷通过改变肿瘤微环境促进BRCA熟练的乳腺癌生长
聚(ADP-核糖)聚合酶-1(PARP1)和梳压抑复合物2(PRC2)各自已知的用于癌症的个体的角色,但他们的合作作用只在DNA损伤修复过程被研究的背景下BRCA -突变型癌症。在这里,我们显示,在MDA-MB-231 BRCA精通的三阴性乳腺癌(TNBC)细胞系中同时抑制PARP1和PRC2可以导致合成活力,而与DNA损伤修复机制无关。具体来说,我们发现PARP1和PRC2的遗传损耗或药理抑制作用可以加快肿瘤的生长速度。我们将其归因于双重耗尽的乳腺癌细胞在肿瘤微环境(TME)中的修饰,例如促进肿瘤内血管生成和增加肿瘤促进2型(M2)巨噬细胞的比例。这些变化随后抑制细胞死亡并促进增殖。从机理上讲,我们发现PARP1和PRC2的双耗竭不仅会诱导TME中NF-κB途径的基础活化,而且还会在外部刺激(如缺氧和巨噬细胞的存在)的反应中最大程度地激活NF-κB的活化。总而言之,我们的研究揭示了BRCA中PARP1和PRC2之间前所未有的合成可行的相互作用精通TNBC,并将NF-κB识别为下游介体。











































 京公网安备 11010802027423号
京公网安备 11010802027423号