Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Two novel heteropolymer‐forming proteins maintain the multicellular shape of the cyanobacterium Anabaena sp. PCC 7120
The FEBS Journal ( IF 5.5 ) Pub Date : 2020-11-17 , DOI: 10.1111/febs.15630 Benjamin L Springstein 1 , Dennis J Nürnberg 2 , Christian Woehle 1 , Julia Weissenbach 1 , Marius L Theune 1 , Andreas O Helbig 3 , Iris Maldener 4 , Tal Dagan 1 , Karina Stucken 5
The FEBS Journal ( IF 5.5 ) Pub Date : 2020-11-17 , DOI: 10.1111/febs.15630 Benjamin L Springstein 1 , Dennis J Nürnberg 2 , Christian Woehle 1 , Julia Weissenbach 1 , Marius L Theune 1 , Andreas O Helbig 3 , Iris Maldener 4 , Tal Dagan 1 , Karina Stucken 5
Affiliation
|
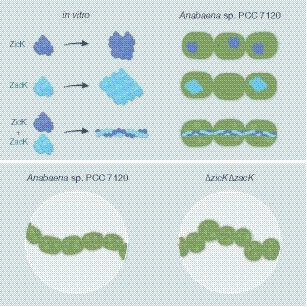
Polymerizing and filament‐forming proteins are instrumental for numerous cellular processes such as cell division and growth. Their function in stabilization and localization of protein complexes and replicons is achieved by a filamentous structure. Known filamentous proteins assemble into homopolymers consisting of single subunits – for example, MreB and FtsZ in bacteria – or heteropolymers that are composed of two subunits, for example, keratin and α/β tubulin in eukaryotes. Here, we describe two novel coiled‐coil‐rich proteins (CCRPs) in the filament‐forming cyanobacterium Anabaena sp. PCC 7120 (hereafter Anabaena) that assemble into a heteropolymer and function in the maintenance of the Anabaena multicellular shape (termed trichome). The two CCRPs – Alr4504 and Alr4505 (named ZicK and ZacK) – are strictly interdependent for the assembly of protein filaments in vivo and polymerize nucleotide independently in vitro, similar to known intermediate filament (IF) proteins. A ΔzicKΔzacK double mutant is characterized by a zigzagged cell arrangement and hence a loss of the typical linear Anabaena trichome shape. ZicK and ZacK interact with themselves, with each other, with the elongasome protein MreB, the septal junction protein SepJ and the divisome associate septal protein SepI. Our results suggest that ZicK and ZacK function in cooperation with SepJ and MreB to stabilize the Anabaena trichome and are likely essential for the manifestation of the multicellular shape in Anabaena. Our study reveals the presence of filament‐forming IF‐like proteins whose function is achieved through the formation of heteropolymers in cyanobacteria.
中文翻译:

两种新的形成杂聚物的蛋白质维持了蓝藻鱼腥藻的多细胞形状。PCC 7120
聚合蛋白和长丝形成蛋白对许多细胞过程(例如细胞分裂和生长)起着重要作用。它们在蛋白质复合物和复制子的稳定和定位中的功能是通过丝状结构实现的。已知的丝状蛋白会组装成由单个亚基组成的均聚物(例如细菌中的MreB和FtsZ),或由两个亚基组成的杂聚物(如真核生物中的角蛋白和α/β微管蛋白)。在这里,我们描述了在长丝形成蓝藻两种新型盘绕富线圈蛋白(CCRPs)鱼腥藻。PCC 7120(以下称“鱼腥藻”)组装成杂聚物并在鱼腥藻的维护中发挥作用多细胞形状(称为毛状体)。类似于已知的中间丝(IF)蛋白质,两个CCRP – Alr4504和Alr4505(分别称为ZicK和ZacK)在体内组装蛋白丝并在体外独立地聚合核苷酸是严格相互依存的。甲Δ济科Δ扎克双突变体的特征在于具有锯齿形电池布置并且因此典型的线性的损失鱼腥毛状体形状。ZicK和ZacK与延伸体蛋白MreB,间隔连接蛋白SepJ和与卵泡缔合的间隔蛋白SepI彼此相互作用。我们的结果表明,ZicK和ZacK与SepJ和MreB共同发挥作用来稳定鱼腥藻。毛状体和可能是必需的在鱼腥藻多细胞形状的表现。我们的研究揭示了长丝形成的IF样蛋白的存在,其功能是通过在蓝细菌中形成杂聚物来实现的。
更新日期:2020-11-17
中文翻译:

两种新的形成杂聚物的蛋白质维持了蓝藻鱼腥藻的多细胞形状。PCC 7120
聚合蛋白和长丝形成蛋白对许多细胞过程(例如细胞分裂和生长)起着重要作用。它们在蛋白质复合物和复制子的稳定和定位中的功能是通过丝状结构实现的。已知的丝状蛋白会组装成由单个亚基组成的均聚物(例如细菌中的MreB和FtsZ),或由两个亚基组成的杂聚物(如真核生物中的角蛋白和α/β微管蛋白)。在这里,我们描述了在长丝形成蓝藻两种新型盘绕富线圈蛋白(CCRPs)鱼腥藻。PCC 7120(以下称“鱼腥藻”)组装成杂聚物并在鱼腥藻的维护中发挥作用多细胞形状(称为毛状体)。类似于已知的中间丝(IF)蛋白质,两个CCRP – Alr4504和Alr4505(分别称为ZicK和ZacK)在体内组装蛋白丝并在体外独立地聚合核苷酸是严格相互依存的。甲Δ济科Δ扎克双突变体的特征在于具有锯齿形电池布置并且因此典型的线性的损失鱼腥毛状体形状。ZicK和ZacK与延伸体蛋白MreB,间隔连接蛋白SepJ和与卵泡缔合的间隔蛋白SepI彼此相互作用。我们的结果表明,ZicK和ZacK与SepJ和MreB共同发挥作用来稳定鱼腥藻。毛状体和可能是必需的在鱼腥藻多细胞形状的表现。我们的研究揭示了长丝形成的IF样蛋白的存在,其功能是通过在蓝细菌中形成杂聚物来实现的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号