当前位置:
X-MOL 学术
›
Int. J. Energy Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Generation of hydrogen upon immersion of Mg in Mn(Ac)2 and the reaction mechanism
International Journal of Energy Research ( IF 4.3 ) Pub Date : 2020-11-17 , DOI: 10.1002/er.6066 Tingting Xu 1 , Ning Wang 2 , Yujun Chai 1
International Journal of Energy Research ( IF 4.3 ) Pub Date : 2020-11-17 , DOI: 10.1002/er.6066 Tingting Xu 1 , Ning Wang 2 , Yujun Chai 1
Affiliation
|
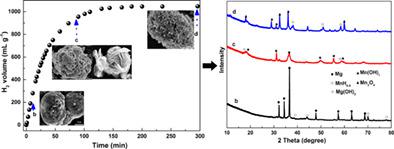
Reactions between metals and H2O could provide high‐purity hydrogen gas for portable fuel cell. However, the initial reaction is typically hindered due to the existence of a dense outer oxide film. Herein, rapid generation of hydrogen following immersion of Mg in a solution of Mn(Ac)2 is examined. The formation of an Mg/Mn galvanic cell results in the destruction of an oxide film, inducing a continuous reaction between Mg, Mn, and water. Moreover, Mn is then oxidized back to Mn2+, illustrating that the metal further participates in the generation of hydrogen. The detected reaction rate varies from 37.5 to 55 mL g−1 minute−1, and the hydrogen volume is ~1000 mL at 35°C. Furthermore, the in‐situ potential changes with the generation of hydrogen, reflecting the fluctuation of the Mg2+ and OH− ions. Notably, the formation of flake‐type Mn or Mn(OH)2 facilitates the permeation of water, OH− ions, and the overflow of hydrogen. Conversely, the accumulation of square‐type Mg(OH)2 hinders permeation, lowering the hydrogen generation rate. The results of the present study provide new insights into the design of highly pure hydrogen generation systems by adjusting the solution composition.
中文翻译:

Mg浸入Mn(Ac)2中时产生的氢及其反应机理
金属与H 2 O之间的反应可为便携式燃料电池提供高纯度氢气。但是,由于存在致密的外部氧化膜,通常会阻碍初始反应。在此,研究了将Mg浸渍在Mn(Ac)2的溶液中后氢的快速生成。Mg / Mn原电池的形成导致氧化膜的破坏,引起Mg,Mn和水之间的连续反应。此外,Mn然后被氧化回Mn 2+,说明该金属进一步参与了氢的产生。检测到的反应速率从37.5到55 mL g -1分钟-1,在35°C下氢气量为〜1000 mL。此外,原位电位产生氢气的变化,反映了镁的波动2+和OH -离子。值得注意的是,薄片型的Mn或Mn的形成(OH)2有利于水,OH的渗透-离子和氢气的溢出。相反,方形Mg(OH)2的积累会阻碍渗透,降低氢的生成速率。通过调节溶液组成,本研究的结果为高纯度制氢系统的设计提供了新的见识。
更新日期:2020-11-17
中文翻译:

Mg浸入Mn(Ac)2中时产生的氢及其反应机理
金属与H 2 O之间的反应可为便携式燃料电池提供高纯度氢气。但是,由于存在致密的外部氧化膜,通常会阻碍初始反应。在此,研究了将Mg浸渍在Mn(Ac)2的溶液中后氢的快速生成。Mg / Mn原电池的形成导致氧化膜的破坏,引起Mg,Mn和水之间的连续反应。此外,Mn然后被氧化回Mn 2+,说明该金属进一步参与了氢的产生。检测到的反应速率从37.5到55 mL g -1分钟-1,在35°C下氢气量为〜1000 mL。此外,原位电位产生氢气的变化,反映了镁的波动2+和OH -离子。值得注意的是,薄片型的Mn或Mn的形成(OH)2有利于水,OH的渗透-离子和氢气的溢出。相反,方形Mg(OH)2的积累会阻碍渗透,降低氢的生成速率。通过调节溶液组成,本研究的结果为高纯度制氢系统的设计提供了新的见识。











































 京公网安备 11010802027423号
京公网安备 11010802027423号