当前位置:
X-MOL 学术
›
Chem. Biodivers.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Efficient and short method for conjugation of 1‐nitro‐9‐aminoacridine to important peptidyl fragments by a solid support synthesis
Chemistry & Biodiversity ( IF 2.3 ) Pub Date : 2020-12-22 , DOI: 10.1002/cbdv.202000702 Monika Lahutta 1
Chemistry & Biodiversity ( IF 2.3 ) Pub Date : 2020-12-22 , DOI: 10.1002/cbdv.202000702 Monika Lahutta 1
Affiliation
|
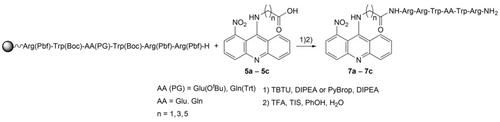
The efficient and short techniques for conjugation of 9-aminoacridine with different peptidyl fragments are necessary for the development of active pharmaceutical ingredients (API). They need to be adopted to generate a new branch of acridine conjugates, enhancing their bioavailability for the examination in biological systems. The branch of developing acridine conjugates, built via different linkers and synthesized in this study, are expected as potential effective chemotherapeutics with dual mechanism of action. Recently, the methodology based on a solid-phase technique has been successfully demonstrated in preparing a number of promising compounds. However, the reaction conditions for amide bond formation between 1-nitro-9-aminoacridine and peptidyl fragments need to be optimized. In this study, the optimization of amide bond formation was demonstrated with the use of the solid-phase synthesis to build a new promising group of 1-nitro-9-aminoacridines conjugated to lactoferrin fragments via especially carboxy linker length.
中文翻译:

通过固体支持物合成将 1-硝基-9-氨基吖啶与重要肽基片段缀合的有效且简短的方法
将 9-氨基吖啶与不同肽基片段结合的高效且简短的技术是开发活性药物成分 (API) 所必需的。需要采用它们来生成吖啶结合物的新分支,从而提高它们在生物系统中检查的生物利用度。通过不同的接头构建并在本研究中合成的开发吖啶缀合物的分支有望成为具有双重作用机制的潜在有效化学治疗剂。最近,基于固相技术的方法已在制备许多有前景的化合物方面得到成功证明。然而,1-硝基-9-氨基吖啶和肽基片段之间形成酰胺键的反应条件需要优化。在这项研究中,
更新日期:2020-12-22
中文翻译:

通过固体支持物合成将 1-硝基-9-氨基吖啶与重要肽基片段缀合的有效且简短的方法
将 9-氨基吖啶与不同肽基片段结合的高效且简短的技术是开发活性药物成分 (API) 所必需的。需要采用它们来生成吖啶结合物的新分支,从而提高它们在生物系统中检查的生物利用度。通过不同的接头构建并在本研究中合成的开发吖啶缀合物的分支有望成为具有双重作用机制的潜在有效化学治疗剂。最近,基于固相技术的方法已在制备许多有前景的化合物方面得到成功证明。然而,1-硝基-9-氨基吖啶和肽基片段之间形成酰胺键的反应条件需要优化。在这项研究中,











































 京公网安备 11010802027423号
京公网安备 11010802027423号