当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Indanones and Spiroindanones by Diastereoselective Annulation Based on a Hydrogen Autotransfer Strategy
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-11-17 , DOI: 10.1002/anie.202013792 Yate Chen 1 , Zhengtian Ding 1 , Yiming Wang 1 , Wenfeng Liu 1 , Wangqing Kong 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-11-17 , DOI: 10.1002/anie.202013792 Yate Chen 1 , Zhengtian Ding 1 , Yiming Wang 1 , Wenfeng Liu 1 , Wangqing Kong 1
Affiliation
|
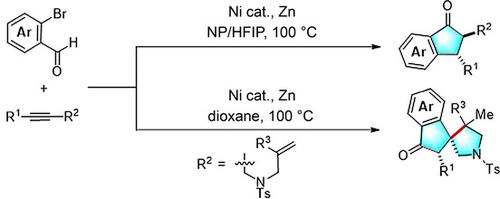
An unprecedented nickel‐catalyzed domino reductive cyclization of alkynes and o‐bromoaryl aldehydes is described. The reaction features broad substrate scope and is tolerant of a variety of functional groups, providing straightforward access to biologically significant indanones and spiroindanone pyrrolidine derivatives in good yields with excellent regio‐ and diastereoselectivity. Preliminary mechanistic studies have shown that indanones are formed by the cyclization of o‐bromoaryl aldehydes and alkynes to form indenol intermediates, followed by hydrogen autotransfer.
中文翻译:

基于氢自动转移策略的非对映选择性环合合成茚满酮和螺并茚满酮
描述了前所未有的镍催化炔和邻溴代芳基醛的多米诺还原环化反应。该反应具有广泛的底物范围,可耐受各种官能团,可直接获得具有生物学意义的茚满酮和螺并茚满酮吡咯烷衍生物,并具有良好的收率和优异的区域选择性和非对映选择性。初步的机理研究表明,茚满酮是由邻溴代芳基醛和炔烃环化形成茚满中间体而形成的,然后氢自动转移。
更新日期:2020-11-17
中文翻译:

基于氢自动转移策略的非对映选择性环合合成茚满酮和螺并茚满酮
描述了前所未有的镍催化炔和邻溴代芳基醛的多米诺还原环化反应。该反应具有广泛的底物范围,可耐受各种官能团,可直接获得具有生物学意义的茚满酮和螺并茚满酮吡咯烷衍生物,并具有良好的收率和优异的区域选择性和非对映选择性。初步的机理研究表明,茚满酮是由邻溴代芳基醛和炔烃环化形成茚满中间体而形成的,然后氢自动转移。











































 京公网安备 11010802027423号
京公网安备 11010802027423号