当前位置:
X-MOL 学术
›
J. Chem. Thermodyn.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Time-temperature-transformation of BiFeO3 phase synthesized by citrate-nitrate route and a synergetic effect for its stabilization
The Journal of Chemical Thermodynamics ( IF 2.2 ) Pub Date : 2021-05-01 , DOI: 10.1016/j.jct.2020.106347 Shama Parwin , Jayanta Parui
The Journal of Chemical Thermodynamics ( IF 2.2 ) Pub Date : 2021-05-01 , DOI: 10.1016/j.jct.2020.106347 Shama Parwin , Jayanta Parui
|
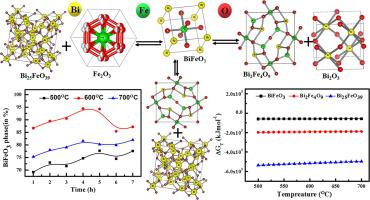
Abstract BiFeO3 has been synthesized from the nitrate salts of bismuth and iron at different temperatures at (500, 600 and 700) °C. The time-temperature-transformation (TTT) has been analysed at the annealing time duration of (1, 2, 3, 4, 5, 6 and 7) h. Thermo-gravimetric study of the starting solid has been analysed from room temperature to 800 °C and the reaction kinetics have been investigated from (400 to 700) °C using Flynn-Wall-Ozawa model which has also been correlated with the differential thermal analysis (DTA). Gibbs energies of the formation of BiFeO3, Bi2Fe4O9 and Bi25FeO39 have been calculated to investigate the reason for the maximum percentage of pure BiFeO3 phase formation at 600 °C. Based on the calculation, though later two compounds are thermodynamically destabilizing impurities of BiFeO3, its synergetic stabilization has been supported by the diffusion kinetics and their activation energies.
中文翻译:

柠檬酸-硝酸盐路线合成的 BiFeO3 相的时间-温度-转变及其稳定的协同作用
摘要 BiFeO3 由铋和铁的硝酸盐在(500、600 和 700)°C 的不同温度下合成。已在 (1, 2, 3, 4, 5, 6 和 7) h 的退火持续时间内分析了时间-温度-转变 (TTT)。已从室温到 800 °C 分析了起始固体的热重研究,并使用 Flynn-Wall-Ozawa 模型研究了(400 到 700)°C 的反应动力学,该模型也与差热分析相关(DTA)。已经计算了 BiFeO3、Bi2Fe4O9 和 Bi25FeO39 形成的吉布斯能量,以研究在 600°C 时纯 BiFeO3 相形成百分比最大的原因。根据计算,虽然后面的两种化合物是 BiFeO3 的热力学不稳定杂质,
更新日期:2021-05-01
中文翻译:

柠檬酸-硝酸盐路线合成的 BiFeO3 相的时间-温度-转变及其稳定的协同作用
摘要 BiFeO3 由铋和铁的硝酸盐在(500、600 和 700)°C 的不同温度下合成。已在 (1, 2, 3, 4, 5, 6 和 7) h 的退火持续时间内分析了时间-温度-转变 (TTT)。已从室温到 800 °C 分析了起始固体的热重研究,并使用 Flynn-Wall-Ozawa 模型研究了(400 到 700)°C 的反应动力学,该模型也与差热分析相关(DTA)。已经计算了 BiFeO3、Bi2Fe4O9 和 Bi25FeO39 形成的吉布斯能量,以研究在 600°C 时纯 BiFeO3 相形成百分比最大的原因。根据计算,虽然后面的两种化合物是 BiFeO3 的热力学不稳定杂质,











































 京公网安备 11010802027423号
京公网安备 11010802027423号