Chemistry of Heterocyclic Compounds ( IF 1.5 ) Pub Date : 2020-11-18 , DOI: 10.1007/s10593-020-02815-0 Igor B. Kutyashev , Ivan А. Kochnev , Anastasiya А. Cherepkova , Nikolay S. Zimnitskiy , Alexey Yu. Barkov , Vladislav Yu. Korotaev , Vyacheslav Ya. Sosnovskikh
|
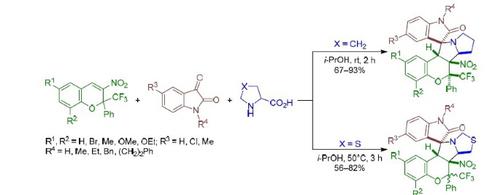
1,3-Dipolar cycloaddition of stabilized azomethine ylides generated in situ from isatins and proline to 3-nitro-2-phenyl-2-trifluoromethyl-2H-chromenes in i-PrOH proceeds stereoselectively at room temperature and leads to the formation of hexahydro-6H-spiro[chromeno[3,4-a]-pyrrolizine-11,3'-indolin]-2'-ones with the cis arrangement of the trifluoromethyl group and the nitro group. A similar reaction with the participation of thiaproline-based ylides at 50°С leads to mixtures of diastereomeric tetrahydro-6H,9H-spiro[chromeno[3',4':3,4]pyrrolo[1,2-c]thiazole-11,3'-indolin]-2'-ones with a predominance of the cis- or trans-isomer. The stereochemistry of the obtained products was confirmed by the NOESY experiment and X-ray structural analysis.
中文翻译:

3-硝基-2-苯基-2-三氟甲基-2 H-色烯与靛红和(硫代)脯氨酸的甲亚胺烷基化物反应:合成螺[铬(硫代)吡咯烷嗪-11,3'-羟吲哚]
从isatins和脯氨酸到i -PrOH中的3-硝基-2-苯基-2-三氟甲基-2 H-色烯就地生成的稳定的偶氮甲亚胺的1,3-偶极环加成反应在室温下立体选择性地进行并导致六氢具有三氟甲基和硝基的顺式排列的-6 H-螺环[ chromeno [3,4- a ]-吡咯嗪-11,3'-吲哚啉] -2'-一。在50°C时有基于噻脯氨酸的基团参与的类似反应导致非对映异构体四氢-6 H,9 H-螺[chromeno [3',4':3,4] pyrrolo [1,2- c ]的混合物噻唑-11,3'-吲哚啉] -2'-占主导地位顺式或反式异构体。通过NOESY实验和X射线结构分析证实了所得产物的立体化学。



























 京公网安备 11010802027423号
京公网安备 11010802027423号